Introduction
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
Houston Methodist Dr. Mary and Ron Neal Cancer Center
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
George and Angelina Kostas Research Center for Cardiovascular Medicine
New Endowed Chairs Positions
EnMed
Center for Bioenergetics
result
Clinical Research
Outcomes, Quality and Healthcare Performances
Restorative Medicine
Precision Medicine
Science in Service
of
Medicineresult
President's letter
2022 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

George and Angelina Kostas Research Center for Cardiovascular Medicine

New Endowed Chairs Positions

EnMed

Center for Bioenergetics

From Discovery to Clinic


What is "Discovery to Clinic"?

Clinical Research


Houston Methodist Conducts First-Ever Study into a Challenging Situation

Can Regulating Cellular Aging Mitigate Both Cancer and Heart Disease?

Innovative Treatment for Chronic Rhinitis is Safe and Effective


Masters of Disguise: Glioblastomas Trick the Immune System by Masquerading as Reproductive Tissue
Improved Options for Patients with Severe Retinal Vascular Disease

A New FDA-Approved Treatment for Sufferers of Chronic Constipation

Houston Methodist joins the Gulf Coast Consortia

Outcomes, Quality and Healthcare Performance


New Findings on RNA Helicases May Yield New Intestinal Disease Therapy

Houston Methodist and Pennsylvania State University Collaborate on a Smartphone App That Could Revolutionize Stroke Diagnosis

New Frontiers to Improve Cardiovascular Medicine and Disease Management

Ongoing Lessons in a Pandemic

Transplants can Boost Survival Rate of Patients with Unresectable Liver Cancers

Telehealth Video Visits During the COVID-19 Pandemic – a Glimpse into the Future?

SARS-CoV-2 Induced Chronic Oxidative Stress and Endothelial Cell Inflammation May Increase Likelihood of Cardiovascular Diseases and Respiratory Failure

Restorative Medicine


Lessening Pain After Knee Replacement Surgery

Do Motor Neurons First Die in the Brain? Study Provides Clues about ALS Origins

Bringing Back Hand Function in People with Complete Spinal Cord Injury

Novel Vascular Engineering Platforms Are a Boon for Bioengineering

Ultra-high-Resolution Scanner Reveals if Knee Injury Advances to Osteoarthritis

Houston Methodist Model Demonstrates Reversal from Heart Failure State, Creating the Potential for Innovative Treatment Avenues

Precision Medicine


Rapidly Scalable, All-Inducible Neural Organoids Could Facilitate Drug Screening for Neurological Diseases

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

COVID-19 Infection in Crucial Brain Regions May Lead To Accelerated Brain Aging

Interleukin 9 Secreting Polarized T Cells Show Potential in Solid and Liquid Tumor Treatment

The NanoLymph: Implantable. Adaptable. Anti-cancer


The NanoLymph: Implantable. Adaptable. Anti-cancer.
An Implantable Nanolymph Cancer Vaccine Device can locally recruit and activate antitumor immune cells to elicit a systemic antitumor immune response.
Cancer immunotherapy has revolutionized cancer treatment, but many patients don’t respond, and the approach has limited success in most solid tumors. Immunotherapy resistance can also lead to cancer relapse. Therefore, there is a critical need for boosting the response rate to cancer immunotherapy for all types of cancer and all patient populations. This is where cancer vaccines can play a crucial role. By increasing immunogenicity and maintaining specificity, cancer vaccines could be a more effective approach to eliminate tumors and evade tumor-induced immune suppression.
To be effective, cancer vaccines must be able to identify tumor cells without inducing autoimmunity and must generate widespread antitumor immunity without systemic toxicity. Cancer vaccine strategies targeting dendritic cells (DCs) — powerful antigen presenting cells — have been only minimally effective, while ex vivo strategies are prone to immune rejection and low tissue uptake with patient adherence to protocols difficult to achieve. An alternative to previous approaches is biomaterial-based cancer vaccines, which have been shown to generate antigen-specific responses that boost the cancer-immunity cascade. Several of these systems have been developed, but they are limited to single administration and cannot be tailored as needed to a patient’s ongoing response to the therapy.

Alessandro Grattoni, PhD

Corrine Chua, PhD
Led by Houston Methodist researchers Alessandro Grattoni, PhD, the Frank J. and Jean Raymond Centennial Chair and Professor of Nanomedicine, Chair, Department of Nanomedicine, and Corrine Chua, PhD, Assistant Professor of Nanomedicine, have developed an implantable cancer vaccine device called “NanoLymph” that can recruit and activate antitumor immune cells locally to elicit a systemic antitumor immune response. The device would be implanted subcutaneously and consists of a dual reservoir to hold immune stimulants and antigens. After the incision has healed and any immune response to the foreign body has resolved, the device would be loaded transcutaneously with immunostimulants and hydrogel-encapsulated antigen in the respective reservoirs. The immune stimulants would then be eluted across a nanoporous membrane into the antigen chamber, forming a drug-antigen gradient within the device that would trigger local recruitment of DCs. These DCs would interact with the encapsulated antigens in the device, then head to local lymph nodes to generate a sustained systemic antitumor T cell response. Unlike other cancer vaccine approaches, NanoLymph is designed to be a long-term platform that can be refilled with minimal invasiveness to provide continued immune activation that can be tailored based on a patient’s ongoing response to the therapy.
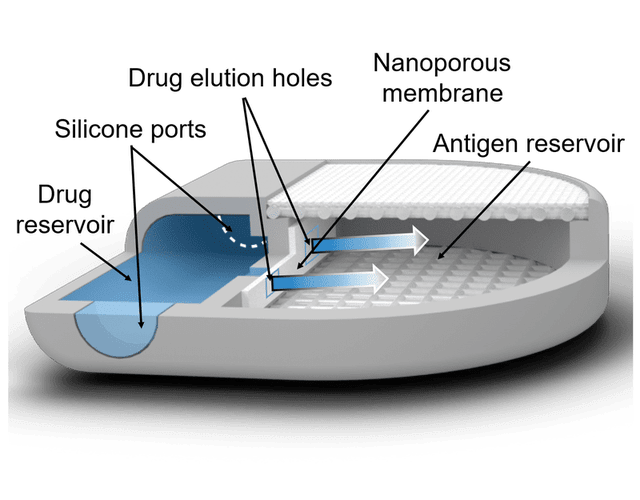
Rendering of NanoLymph cross-section with view of drug and antigen reservoir as well as self-sealing silicone loading ports on opposing sides. Adapted from Viswanath, D.I. et al., (2022) Engineered implantable vaccine platform for continuous antigen-specific immunomodulation. Biomaterials, Feb(281)
Because of its considerable flexibility, the NanoLymph could also be utilized to effectively manage other diseases modulated by the immune system. For example, the NanoLymph could be leveraged for allergy immunotherapy to desensitize patients against common life-threatening allergens. Specifically, the NanoLymph could be used to elute cytokines or mTOR inhibitors, with house dust mite allergoid as the antigen. In this context, the NanoLymph could induce allergen desensitization by tolerogenic DCs (immuno-suppressive dendritic cells) suppressing Th2-mediated immune responses. Most available medical implants cannot be refilled and rely on either complete structure degradation or removal and reimplantation, posing a significant barrier to providing continued care.
With this publication, Grattoni and his team present a proof-of-concept study demonstrating that drug and antigen locally released from the NanoLymph in a spatiotemporal manner successfully recruits and activates DCs to induce an antigen-specific antitumor immune response. Future studies will evaluate the prophylactic and therapeutic efficacy of the antitumor immune response for cancer treatment. This work lays the foundation for development of an immunomodulatory niche that could significantly improve success of cancer immunotherapy and is also adaptable for multiple disease etiologies.
More from Discovery to Clinic