Introduction
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
Houston Methodist Dr. Mary and Ron Neal Cancer Center
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
George and Angelina Kostas Research Center for Cardiovascular Medicine
New Endowed Chairs Positions
EnMed
Center for Bioenergetics
result
Clinical Research
Outcomes, Quality and Healthcare Performances
Restorative Medicine
Precision Medicine
Science in Service
of
Medicineresult
President's letter
2022 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

George and Angelina Kostas Research Center for Cardiovascular Medicine

New Endowed Chairs Positions

EnMed

Center for Bioenergetics

From Discovery to Clinic


What is "Discovery to Clinic"?

Clinical Research


Houston Methodist Conducts First-Ever Study into a Challenging Situation

Can Regulating Cellular Aging Mitigate Both Cancer and Heart Disease?

Innovative Treatment for Chronic Rhinitis is Safe and Effective


Masters of Disguise: Glioblastomas Trick the Immune System by Masquerading as Reproductive Tissue
Improved Options for Patients with Severe Retinal Vascular Disease

A New FDA-Approved Treatment for Sufferers of Chronic Constipation

Houston Methodist joins the Gulf Coast Consortia

Outcomes, Quality and Healthcare Performance


New Findings on RNA Helicases May Yield New Intestinal Disease Therapy

Houston Methodist and Pennsylvania State University Collaborate on a Smartphone App That Could Revolutionize Stroke Diagnosis

New Frontiers to Improve Cardiovascular Medicine and Disease Management

Ongoing Lessons in a Pandemic

Transplants can Boost Survival Rate of Patients with Unresectable Liver Cancers

Telehealth Video Visits During the COVID-19 Pandemic – a Glimpse into the Future?

SARS-CoV-2 Induced Chronic Oxidative Stress and Endothelial Cell Inflammation May Increase Likelihood of Cardiovascular Diseases and Respiratory Failure

Restorative Medicine


Lessening Pain After Knee Replacement Surgery

Do Motor Neurons First Die in the Brain? Study Provides Clues about ALS Origins

Bringing Back Hand Function in People with Complete Spinal Cord Injury

Novel Vascular Engineering Platforms Are a Boon for Bioengineering

Ultra-high-Resolution Scanner Reveals if Knee Injury Advances to Osteoarthritis

Houston Methodist Model Demonstrates Reversal from Heart Failure State, Creating the Potential for Innovative Treatment Avenues

Precision Medicine


Rapidly Scalable, All-Inducible Neural Organoids Could Facilitate Drug Screening for Neurological Diseases

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

COVID-19 Infection in Crucial Brain Regions May Lead To Accelerated Brain Aging

Interleukin 9 Secreting Polarized T Cells Show Potential in Solid and Liquid Tumor Treatment

The NanoLymph: Implantable. Adaptable. Anti-cancer


Designer Nanoparticles Deliver Drug to Dial Down Inflammation

When actor Christopher Reeve, famous for playing Superman, sustained a serious neck injury from falling from a horse, treatment options were few, leaving him and others with traumatic spinal cord injuries with a hugely compromised quality of life. However, current medical advancements across several disciplines, including neurosurgery, nanomedicine and physical therapy, hold promise to improve the standard of care and recovery for people with spinal cord injuries.
Joining the efforts in targeted therapies for spinal cord injury, Houston Methodist researchers have engineered a superparamagnetic nanoparticle that can be directed to go to the site of spinal damage and release a drug to dial down inflammation and promote repair.
“There are beneficial aspects of inflammation, and there are inhibitory effects,” said Philip J. Horner, PhD, Scientific Director, Center for Neurogeneration and Professor of Neuroregeneration and principal investigator on the study, Modulation of Neuroinflammation via Selective Nanoparticle-Mediated Drug Delivery to Activated Microglia/Macrophages in Spinal Cord Injury. “Our aim in this study was to prevent the negative effects of inflammation by combining biochemical pathway and cellular targeting, in other words, target a specific proinflammatory signaling pathway within a specific immune cell type.”
The researchers have published their work in the journal Advanced Therapeutics.
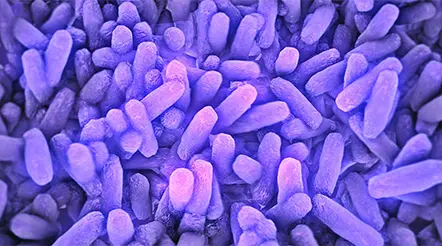
An electron micrograph of the nanoparticle designed by the researchers. Scale bar: 100nm.
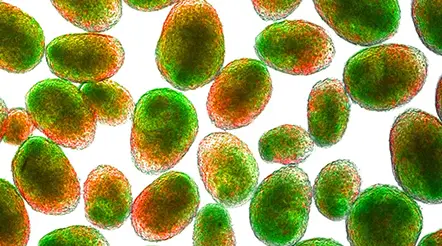
Nanoparticles (red) are internalized in activated macrophages (green) at injury site. Blue marks other cells at the injury site.
According to the World Health Organization, 250,000 to 500,000 individuals worldwide suffer a spinal cord injury each year. Like with any other injury, an insult to the spinal cord sets off a series of repair mechanisms; innate immune cells, such as microglia and macrophages, invade the wounded area and work on restoring tissue homeostasis. However, for spinal cord injuries, this acute inflammation often gives way to a more long-term condition.
“Neuroinflammation plays a crucial step in the recovery process after an injury,” said Horner. “But just after the spinal injury, during the acute injury setting, immune amplification can become a problem, which could then lead to chronic inflammation.”
When chronic inflammation sets in, the immune system’s repair responses get eclipsed by proinflammatory mechanisms that are continuously ramped by the release of cytokines, chemokines and reactive oxygen species by different cells at the injury site. Further, chronic inflammation can last for months, even years.
To prevent an immune run-off to chronic inflammation, the researchers tested if nanoparticles or molecules with diameters less than 100 nanometers could be used to target a proinflammatory phenotype of macrophages during the acute injury setting. They turned their attention to the p38 MAP kinase stress-mediated pathway in these cells wherein the activation of MAPK-activated protein kinase 2 (MK2) causes proinflammatory cytokines release.
“We wanted to selectively target this kinase to turn down the production of proinflammatory cytokines without interfering with the macrophages producing anti-inflammatory cytokines that help with regeneration,” said Cinzia Stigliano, PhD, Research Associate in the Center for Neuroregeneration and the lead author on the study.
For their experiments, Horner and his team surgically induced a hemi-contusion injury in the spinal cord in rodents. Then, into the lateral ventricle, Stigliano injected fluorescently tagged nanoparticles carrying an MK2 inhibitor, PF3644022. Since the designer nanoparticles are superparamagnetic, she could guide them to the injury site magnetically. When she analyzed images of the injury site, she found that the nanoparticles were successfully taken up by the macrophages at the site and, consequently, the levels of proinflammatory cytokines were lowered. She also observed reduced trafficking and accumulation of phagocytic cells, a macrophage phenotype involved in the amplification of inflammatory responses.
With the proof of concept that the nanoparticles can be used to reduce ongoing inflammation, the researchers plan to investigate if this change influences recovery from injury.
“We now have nanoparticles that can be easily produced and injected to target immune cells in an acute injury setting,” said Stigliano. “This study is just the beginning. Next, we would like to combine this technique with other strategies to eventually improve regeneration.”
This research is supported by the Morton Cure Paralysis Fund, the TIRR Foundation through Mission Connect Jerry Johnston Andrew the Craig H. Nielsen Foundation.
More from Discovery to Clinic