Introduction
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
Houston Methodist Dr. Mary and Ron Neal Cancer Center
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
George and Angelina Kostas Research Center for Cardiovascular Medicine
New Endowed Chairs Positions
EnMed
Center for Bioenergetics
result
Clinical Research
Outcomes, Quality and Healthcare Performance
Restorative Medicine
Precision Medicine
Science in Service
of
Medicineresult
President's letter
2022 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

George and Angelina Kostas Research Center for Cardiovascular Medicine

New Endowed Chairs Positions

EnMed

Center for Bioenergetics

From Discovery to Clinic


What is "Discovery to Clinic"?

Clinical Research


Houston Methodist Conducts First-Ever Study into a Challenging Situation

Can Regulating Cellular Aging Mitigate Both Cancer and Heart Disease?

Innovative Treatment for Chronic Rhinitis is Safe and Effective


Masters of Disguise: Glioblastomas Trick the Immune System by Masquerading as Reproductive Tissue
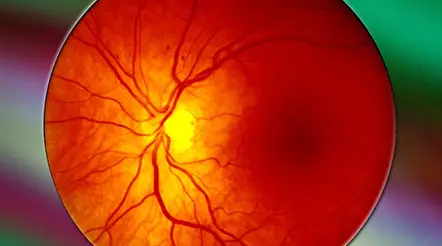
Improved Options for Patients with Severe Retinal Vascular Disease

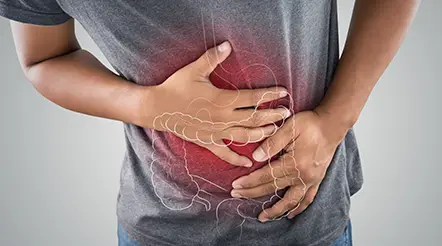
A New FDA-Approved Treatment for Sufferers of Chronic Constipation

Houston Methodist joins the Gulf Coast Consortia

Outcomes, Quality and Healthcare Performance


New Findings on RNA Helicases May Yield New Intestinal Disease Therapy

Houston Methodist and Pennsylvania State University Collaborate on a Smartphone App That Could Revolutionize Stroke Diagnosis

New Frontiers to Improve Cardiovascular Medicine and Disease Management

Ongoing Lessons in a Pandemic

Transplants can Boost Survival Rate of Patients with Unresectable Liver Cancers

Telehealth Video Visits During the COVID-19 Pandemic – a Glimpse into the Future?

SARS-CoV-2 Induced Chronic Oxidative Stress and Endothelial Cell Inflammation May Increase Likelihood of Cardiovascular Diseases and Respiratory Failure

Restorative Medicine


Lessening Pain After Knee Replacement Surgery

Do Motor Neurons First Die in the Brain? Study Provides Clues about ALS Origins

Bringing Back Hand Function in People with Complete Spinal Cord Injury

Novel Vascular Engineering Platforms Are a Boon for Bioengineering

Ultra-high-Resolution Scanner Reveals if Knee Injury Advances to Osteoarthritis

Houston Methodist Model Demonstrates Reversal from Heart Failure State, Creating the Potential for Innovative Treatment Avenues

Precision Medicine


Rapidly Scalable, All-Inducible Neural Organoids Could Facilitate Drug Screening for Neurological Diseases

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

COVID-19 Infection in Crucial Brain Regions May Lead To Accelerated Brain Aging

Interleukin 9 Secreting Polarized T Cells Show Potential in Solid and Liquid Tumor Treatment

The NanoLymph: Implantable. Adaptable. Anti-cancer


To dodge the wrath of the immune system, glioblastoma multiforme — the most diagnosed brain cancer in adults — has a cunning disguise. By producing proteins that are usually reserved for the testicles, semen and placenta, the cancer pulls off a one-of-a-kind con by posing as an outlandish reproductive tissue, deceiving the immune machinery into protection rather than attack.
While unusual in its choice of mimicry, glioblastoma multiforme is no different than other cancers in its persistent attempts at circumventing the immune system. But how this aggressive cancer performs its trickery has been a mystery and an outstanding question in combating the disease.
Towards this end, a team of researchers overseen by David S. Baskin, MD, FAANS, FACS, an internationally recognized clinician scientist and the Director of the Peak Brain Tumor Center, has been unraveling how glioblastoma multiforme mimics reproductive tissue to hijack the immune system.
“Evading the immune system is very difficult, and one of the tricks to success is immuno-camouflage, which the glioblastoma multiforme has mastered,” said Martyn Sharpe, PhD, the John S. “Steve” Dunn Jr. Distinguished Professor in Brain Tumor Research, Asscoiate Research Professor in the Department of Neurosurgery and lead author on the study. “But figuring out how is very complicated – each patient is different; the immune system in men and women is different.”
David Baskin, MD
Martyn Sharpe, PhD
A documented history of glioblastoma multiforme goes back 200 years with German pathologist Rudolf Virchow first naming malignant tumors originating from non-neuronal cells as gliomas. With more advances in histological methods towards the late 19th century, these cells, known as glia, were found to be diverse, consisting of astrocytes, ependymal cells and oligodendrocytes, thereby suggesting a commensurate heterogeneity in glioma subtypes. Among the different gliomas, American physicians Percival Bailey and Harvey Cushing in 1926 classified the most malignant and unusual form of glioma as spongioblastoma multiforme, which over time became known as glioblastoma multiforme, or simply glioblastoma, an aggressive form of central nervous system cancer of astrocytic origin.
Studies show that gonadal hormones or sex steroids and glioblastoma are closely related. For example, the risk of developing glioblastoma is lower in women who have taken contraceptives or have had hormone replacement therapy. Further, patients with glioblastoma often have higher circulating testosterone. The cancer also synthesizes sex steroids, which it uses to promote its malignancy.
Growing glioblastomas compromise the blood-brain barrier: Circulating immune cells that are normally shunned away from the central nervous system can permeate the brain, and among these trespassers, regulatory T cells (Tregs) that are immune-suppressive have been linked to poor outcomes of glioblastoma patients.
But how does the glioblastoma manipulate immune cells to favor its growth? And how do sex steroids facilitate this process?
To answer this question, Sharpe and his team conducted an in-depth analysis of four transcriptome databases to look for genes that were upregulated or downregulated in glioblastoma and then related this information to patient outcomes. In addition, they also searched for genes associated with immune cell populations that might bestow tumor protection.
As a starting point, the researchers classified glioblastoma as estrogenic, androgenic or asteroidogenic (lacking a significant hormonal signature) based on the expression of genes needed for the synthesis of sex steroids. But this stratification strategy was unsuccessful because some glioblastomas that were producing androgens did not express key genes needed for testosterone production.
The team discovered that instead of making androgen via the conventional or canonical pathway, that is, by converting cholesterol to progesterone and then progesterone to testosterone, some of the tumors were producing dihydrotestosterone, a more potent androgen, from cholesterol and expressing genes reserved for producing androgens through backdoor pathways.
In their next attempt at classifying the glioblastoma, Sharpe and his team grouped the tumors into four steroidogenic phenotypes, contingent this time on whether they produced testosterone and progesterone, estrogen and progesterone, estrogen and dihydrotestosterone, or were asteroidogenic. When they reexamined the levels of gene expression in each of the newly stratified glioblastomas, they found, as per their expectation, that the genetic markers for immune-suppressive cells were elevated. But more strikingly, so were the markers for seminal fluid, sperm and placental proteins.
“Something really interesting and peculiar was going on,” said Amanda Jenson, MD, Chief Resident of Neurosurgery and an author on the study. “Some of the tumors were acting like fetuses by expressing pregnancy-related proteins to evade the immune system.”
The researchers also uncovered that the patients with glioblastoma had the worst outcome if their tumors expressed sperm-specific proteins. While this result is intuitive for men since they produce male proteins, it was a conundrum for women having glioblastoma. In other words, why would women do worse if their tumors expressed male-specific proteins?
Searching for clues, Sharpe and his team took a deep dive into the scientific literature and uncovered that the answer to their seemingly bizarre findings had roots in the converging tales of the immune and reproductive system.
Early in life, the adaptive immune system learns to recognize items that belong to “self” versus “non-self.” Briefly, naïve CD4+ T cells made in the bone marrow travel to the thymus organ to get educated about proteins that belong uniquely to an individual. Here, they learn to recognize “self” epitopes, or tiny protein tags belonging to specific bodily tissues, by attaching their receptors on major histocompatibility complex II molecules bound to thymic epithelial cells. When there is a match, CD4+ T cells that express receptors for “self” epitopes are either killed or put on a path to becoming non-inflammatory Tregs. Also, B cells with epitopes that match receptors on Tregs are either terminated or driven to become regulatory B cells or Bregs.
While thymic maturation of immune cells is similar for men and women, there are certain differences between the sexes. When boys reach puberty, they produce semen, whose contents are foreign to the immune system. To prevent an inflammatory response from circulating immune cells, the testicles are carefully guarded by the tight junctions formed by Sertoli cells of the blood-testis barrier. These cells present male-specific epitopes on their major histocompatibility complex II molecules. Surveilling CD4+ T cells that recognize the epitopes are instructed to become related orphan receptor C (RORC) Tregs. And B cells expressing semen-specific epitopes recognized by Tregs are killed or become Bregs, resulting in the disappearance of anti-seminal antibodies after puberty.
Glioblastoma is classified as a grade IV astrocytoma.
48% of all primary malignant brain tumors are glioblastomas.
Mean age
at diagnosis is 64.
More than 10,000 individuals in the United States will succumb to glioblastoma every year.
Median overall survival is 14.6 months
Glioblastoma is classified as a grade IV astrocytoma.
48% of all primary malignant brain tumors are glioblastomas.
Mean age
at diagnosis is 64.
More than 10,000 individuals in the United States will succumb to glioblastoma every year.
Median overall survival is 14.6 months
Women, on the other hand, start producing their Tregs for semen- and testicular-specific proteins after coitus. CD4+ T cells at the mucosal lining in the cervix possessing receptors for seminal epitopes are told by the immune system to convert into uterocervical RORC Tregs and to henceforth recognize the contents of semen as privileged, thwarting an inflammatory immune response.
Another set of immune-modifying events occurs when a woman is pregnant. She produces progesterone and the growing baby makes fetal- and placenta-specific proteins, attracting another class of innate immune cells called monocytes. These monocytes then differentiate, transforming into their new avatars as myeloid-derived suppressor cells and tumor-associated macrophages that are anti-inflammatory and protect the baby from the mother’s immune system.
Armed with this knowledge, Sharpe’s team scrutinized the immune cell population infiltrating the glioblastoma and found elevated genetic signatures for RORC Tregs, myeloid-suppressor cells and tumor-associated macrophages. The cancer, it seemed, was hijacking a variety of immune-suppressive cells by expressing a combination of reproductive-associated proteins.
Sharpe explained that glioblastoma, like any other cancer, is transcriptionally hyperactive, constantly switching on silenced genes by demethylating DNA. So, if the genes for seminal proteins that are otherwise silent get activated, then the glioblastoma opens itself to be recognized and protected by RORC Tregs. In women, the activation of seminal proteins draws the uterocervical RORC Tregs towards the glioblastoma. Similarly, if the tumor starts to express placental proteins, it could bind to receptors on myeloid-suppressor cells and tumor-associated macrophages, which would then confer protection.
“There isn't one sperm-specific or placenta-specific protein that causes the Tregs to be attracted, it's a basket of different proteins,” said Sharpe. “A woman, for instance, has Tregs for proteins in her partner’s semen. And as far as the cancer is concerned, it doesn't care which one of the seminal proteins it makes as long as it gets the immunosuppressive effect from the Tregs.”
DNA demethylation may even initiate steroid synthesis serendipitously. And if the genes needed to make androgens turn on, the tumor gets an extra boost since many of the reproductive-associated proteins are controlled either directly or indirectly by male hormones. The researchers’ findings on glioblastoma’s evasion strategy are published in the International Journal of Molecular Sciences.
Sharpe noted that the glioblastoma’s close dependence on sex steroids could offer the opportunity to repurpose hormone therapy-based drugs that are already used in clinics to treat breast and castration-resistant prostate cancer. But a prerequisite for the efficacy of these treatments is identifying the steroidal indication of the cancer, a next step in the team’s efforts.
“These findings shed light on the fact that immunotherapies, like ipilimumab and nivolumab, which are the main immune checkpoint inhibitors, have been effective in many other cancers but have not been successful in treating glioblastoma,” said Baskin. “It is clear that glioblastomas have a unique ability to produce an immunosuppressive environment that cannot be overcome. Until now, this has been a mystery. The current study conceived and executed by our team provides critical clues as to why this is happening and presents a novel pathway for overcoming this immune suppression."
More from Discovery to Clinic