


Introduction
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
Houston Methodist Dr. Mary and Ron Neal Cancer Center
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
George and Angelina Kostas Research Center for Cardiovascular Medicine
New Endowed Chairs Positions
EnMed
Center for Bioenergetics
result
Clinical Research
Outcomes, Quality and Healthcare Performances
Restorative Medicine
Precision Medicine
Science in Service
of
Medicineresult
President's letter
2022 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

George and Angelina Kostas Research Center for Cardiovascular Medicine

New Endowed Chairs Positions

EnMed

Center for Bioenergetics

From Discovery to Clinic


What is "Discovery to Clinic"?

Clinical Research


Houston Methodist Conducts First-Ever Study into a Challenging Situation

Can Regulating Cellular Aging Mitigate Both Cancer and Heart Disease?

Innovative Treatment for Chronic Rhinitis is Safe and Effective


Masters of Disguise: Glioblastomas Trick the Immune System by Masquerading as Reproductive Tissue
Improved Options for Patients with Severe Retinal Vascular Disease

A New FDA-Approved Treatment for Sufferers of Chronic Constipation

Houston Methodist joins the Gulf Coast Consortia

Outcomes, Quality and Healthcare Performance


New Findings on RNA Helicases May Yield New Intestinal Disease Therapy

Houston Methodist and Pennsylvania State University Collaborate on a Smartphone App That Could Revolutionize Stroke Diagnosis

New Frontiers to Improve Cardiovascular Medicine and Disease Management

Ongoing Lessons in a Pandemic

Transplants can Boost Survival Rate of Patients with Unresectable Liver Cancers

Telehealth Video Visits During the COVID-19 Pandemic – a Glimpse into the Future?

SARS-CoV-2 Induced Chronic Oxidative Stress and Endothelial Cell Inflammation May Increase Likelihood of Cardiovascular Diseases and Respiratory Failure

Restorative Medicine


Lessening Pain After Knee Replacement Surgery

Do Motor Neurons First Die in the Brain? Study Provides Clues about ALS Origins

Bringing Back Hand Function in People with Complete Spinal Cord Injury

Novel Vascular Engineering Platforms Are a Boon for Bioengineering

Ultra-high-Resolution Scanner Reveals if Knee Injury Advances to Osteoarthritis

Houston Methodist Model Demonstrates Reversal from Heart Failure State, Creating the Potential for Innovative Treatment Avenues

Precision Medicine


Rapidly Scalable, All-Inducible Neural Organoids Could Facilitate Drug Screening for Neurological Diseases

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

COVID-19 Infection in Crucial Brain Regions May Lead To Accelerated Brain Aging

Interleukin 9 Secreting Polarized T Cells Show Potential in Solid and Liquid Tumor Treatment

The NanoLymph: Implantable. Adaptable. Anti-cancer


Cycle of Translation
A Transformational Vision for Medical Research
Houston Methodist met the challenge of translation early in the design of the Research Institute building. It houses the essential services and technology that support the full cycle of development to efficiently and effectively deliver innovations to the clinic.
Translating laboratory innovations into treatments for patients is fraught with challenges. The lack of specialized translational research resources makes it extremely difficult and expensive for most institutions to turn fundamental discoveries into tangible solutions that benefit the public. Their most promising innovations perish in the "Valley of Death" before they reach the clinic. We provide support at every step of the cycle of translation, from bridge funding to technical expertise with U.S. Food and Drug Administration (FDA)-approved current good manufacturing practice (cGMP) facilities, good laboratory practice (GLP) facilities and clinical trial operations. The most promising innovations have a lifeline at Houston Methodist.
Hover Over Icons to Learn More
Pinch to zoom image
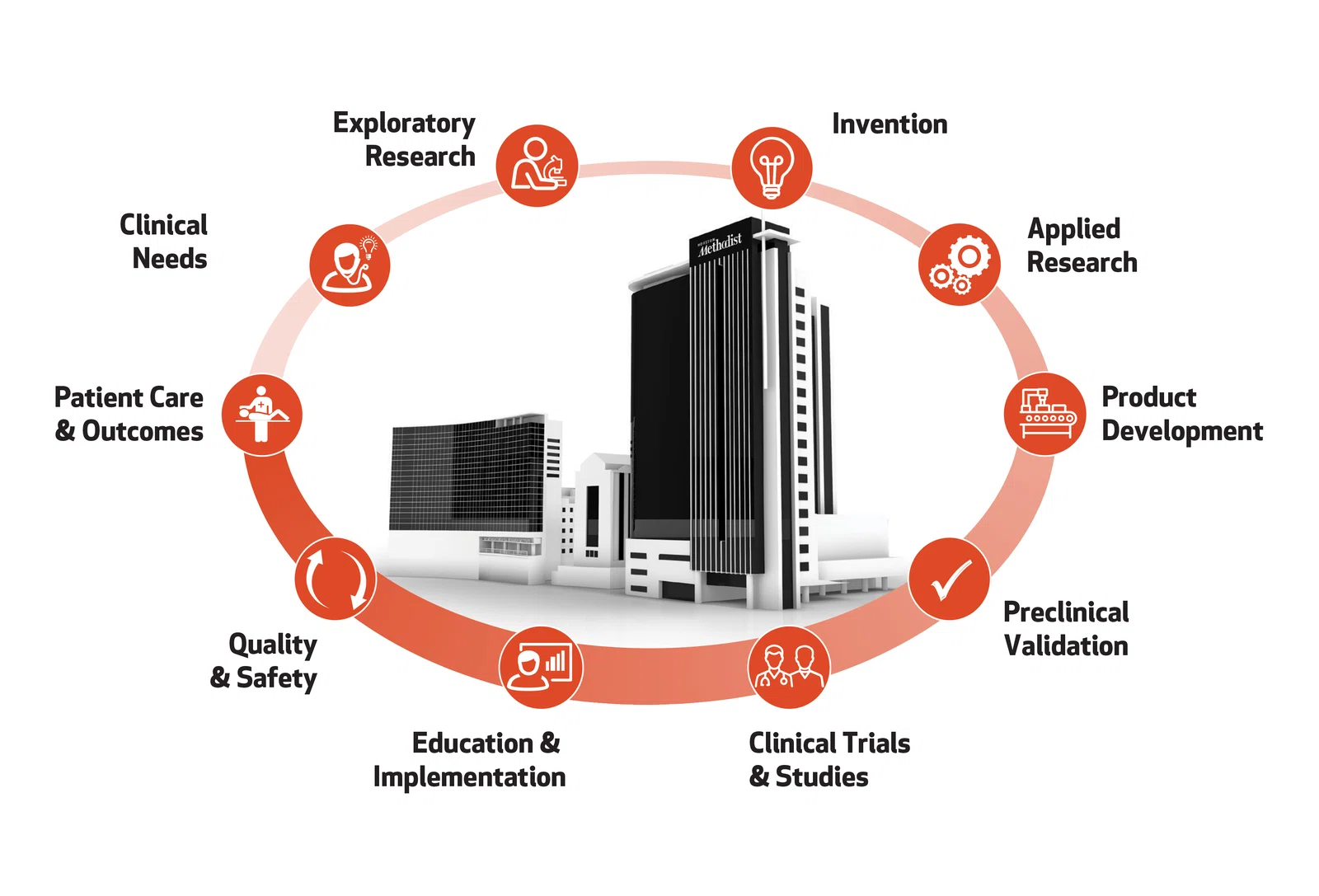

Invention
Inventions that solve a problem have real world value and protectable intellectual property.
Education & Implementation
Interprofessional education programs employ the latest advances in simulation and implementation science to facilitate the safe and efficient adoption of new technologies as they are approved for clinical use.
Quality & Safety
As part of the systemwide goals for quality improvement and patient safety, we develop our education programs to incorporate high reliability organization principles and use team-based training approaches to maximize patient outcomes.
Patient Care & Outcomes
The Cycle begins and ends with the care of patients in our hospitals and clinics. Clinicians in our hospitals care for more than 2.2 million patients annually, enabling them to identify the most pressing challenges in medicine and provide excellent care.
Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.
Product Development
Product development involves the design and piloting of a test product or process within a cGMP or other controlled environment.
Exploratory Research
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process.
Clinical Trials
Teams have access to early phase trial support, pharmacokinetics and pharmacodynamics support and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials.
Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.
Applied Research
Applied research involves testing, evaluating and refining new materials, devices and systems or methods into a final lead experimental product or process with the intent to move into the product development stage.

Product Development
Product development involves the design and piloting of a test product or process within a cGMP or other controlled environment.

Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.

Clinical Trials & Studies
Teams have access to early phase trial support, pharmacokinetics and pharmacodynamics support and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials.

Education & Implementation
Interprofessional education programs employ the latest advances in simulation and implementation science to facilitate the safe and efficient adoption of new technologies as they are approved for clinical use.

Quality & Safety
As part of the systemwide goals for quality improvement and patient safety, we develop our education programs to incorporate high reliability organization principles and use team-based training approaches to maximize patient outcomes.

Patient Care & Outcomes
The Cycle begins and ends with the care of patients in our hospitals and clinics. Clinicians in our hospitals care for more than 2.2 million patients annually, enabling them to identify the most pressing challenges in medicine and provide excellent care.

Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.

Exploratory Research
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process.

Invention
Inventions that solve a problem have real world value and protectable intellectual property.

Applied Research
Applied research involves testing, evaluating and refining new materials, devices and systems or methods into a final lead experimental product or process with the intent to move into the product development stage.













