
President’s Letter
2021 Metrics
Cycle of Translation
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
Houston Methodist Dr. Mary and Ron Neal Cancer Center
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
COVID-19 Studies
Outcomes Research
Restorative Medicine
Houston Methodist Advances Research into Neural Prosthetics
Noninvasive Spinal Stimulation Works to Restore Movement After Spinal Cord Injury
An Innovative Approach to Treat Even the Most Stubborn-to-Heal Fractures
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Houston Methodist, Rice University, Baylor College of Medicine Design Noninvasive Tech to Help Remove Brain’s Metabolic Waste
Houston Methodist Investigators Nanotechnology Investigators Awarded Prestigious Grants from the Department of Defense
Precision Medicine
Cancer Cell Type (Seed) and Tumor Microenvironment (Soil) Control Therapeutic Antibody Delivery and Efficacy
Novel Drug Combination Can Target Triple-Negative Breast Cancer for Treatment
A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine
Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease
New Virtual Intensive Care Unit Simultaneously Improves Patient Care and Bed Capacity
result
Introduction
Joint Weill Cornell–Houston Methodist Academic Institute Doctoral Program Welcomes its Inaugural Class
Visionary EnMed Program Soars to New Heights
Neural Control of Organ Degeneration and Regeneration (NeuralCODR) Training Program
Faculty and Research Development
Graduate Medical Education



Science in Service
of
Medicineresult
President's letter
2021 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

From Discovery to Clinic


What is "Discovery to Clinic"?

Restorative Medicine


Houston Methodist Advances Research into Neural Prosthetics

Noninvasive Spinal Stimulation Works to Restore Movement After Spinal Cord Injury

An Innovative Approach to Treat Even the Most Stubborn-to-Heal Fractures

Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection

Houston Methodist, Rice University, Baylor College of Medicine Design Noninvasive Tech to Help Remove Brain’s Metabolic Waste

Houston Methodist Investigators Nanotechnology Investigators Awarded Prestigious Grants from the Department of Defense

Precision Medicine


Cancer Cell Type (Seed) and Tumor Microenvironment (Soil) Control Therapeutic Antibody Delivery and Efficacy

New Virtual Intensive Care Unit Simultaneously Improves Patient Care and Bed Capacity

Novel Drug Combination Can Target Triple-Negative Breast Cancer for Treatment

A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

Translational Luminaries

Cycle of Translation
A Transformational Vision for Medical Research
Houston Methodist met the challenge of translation early in the design of the Research Institute building. It houses the essential services and technology that support the full cycle of development to efficiently and effectively deliver innovations to the clinic.
Translating laboratory innovations into treatments for patients is fraught with challenges. The lack of specialized translational research resources makes it extremely difficult and expensive for most institutions to turn fundamental discoveries into tangible solutions that benefit the public. Their most promising innovations perish in the "Valley of Death" before they reach the clinic. We provide support at every step of the cycle of translation, from bridge funding to technical expertise with U.S. Food and Drug Administration (FDA)- approved current good manufacturing practice (cGMP) facilities, good laboratory practice (GLP) facilities and clinical trial operations. The most promising innovations have a lifeline at Houston Methodist.
Hover over the icons to learn more
Pinch to zoom image
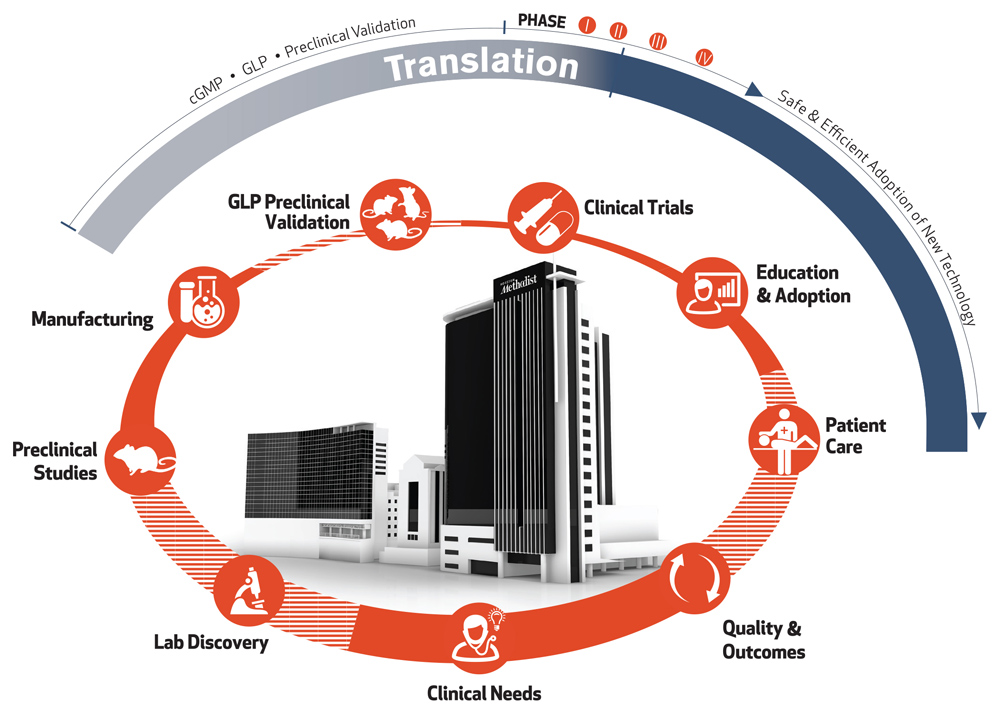
Clinical Trials
Teams have access to early phase trial support (pharmacokinetics and pharmacodynamics), and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials. This is the end phase of the cycle of a cure, opening the door to the future.
Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.
Discovery
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process. Interdisciplinary teams turn ideas into real solutions with sound testing and iterative design.
Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.
Current Good Manufacturing Practice (cGMP) Manufacturing
Houston Methodist is equipped with FDA cGMP-compliant facilities that produce research and clinical-grade therapeutic materials and custom radiopharmaceuticals for preclinical and first-in-human studies.
Patient Care
The cycle begins and ends with the care of patients in our hospitals and clinics. Clinicians in our hospitals care for more than 800,000 patients annually, enabling them to identify the most pressing challenges in medicine and provide excellent care.
Good Laboratory Practice (GLP) Preclinical Validation
The GLP facilities at Houston Methodist perform risk, safety and efficacy assessment studies in compliance with current FDA guidelines in preclinical models. Adherence to GLP standards is required for safety studies in order to move to clinical trials.
Quality & Outcomes
A key component of leading medicine is our culture of quality, safety and innovation. As part of the systemwide goals for quality improvement and patient safety, we develop our education programs to incorporate high reliability organization principles and use team-based training approaches to maximize patient outcomes.
Education & Adoption
Throughout our system, we’re building interprofessional education programs that employ the latest strategies, including simulation, to facilitate the safe and efficient adoption of new technologies as they’re approved for clinical use. Bringing a new innovation from the completion of clinical trials to clinic adoption is very time and resource intensive, needing 10 years and $1 billion, on average.

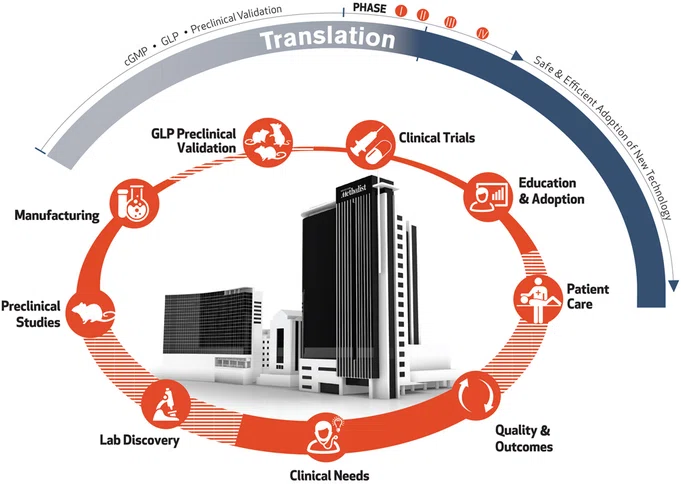
Patient Care
The cycle begins and ends with the care of patients in our hospitals and clinics. Clinicians in our hospitals care for more than 800,000 patients annually, enabling them to identify the most pressing challenges in medicine and provide excellent care.

Quality & Outcomes
A key component of leading medicine is our culture of quality, safety and innovation. As part of the system-wide goals for quality improvement and patient safety, we develop our education programs to incorporate high reliability organization principles and use team-based training approaches to maximize patient outcomes.

Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.

Lab Discovery
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process. Interdisciplinary teams turn ideas into real solutions with sound testing and iterative design.

Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.

Current Good Manufacturing Practice (cGMP) Manufacturing
Houston Methodist is equipped with FDA cGMP-compliant facilities that produce research and clinical-grade therapeutic materials and custom radiopharmaceuticals for preclinical and first-in-human studies.

Good Laboratory Practice (GLP) Preclinical Validation
The GLP facilities at Houston Methodist perform risk, safety and efficacy assessment studies in compliance with current FDA guidelines in preclinical models. Adherence to GLP standards is required for safety studies in order to move to clinical trials.

Clinical Trials
Teams have access to early phase trial support (pharmacokinetics and pharmacodynamics), and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials. This is the end phase of the cycle of a cure, opening the door to the future.

Education & Adoption
Throughout our system, we’re building interprofessional education programs that employ the latest strategies, including simulation, to facilitate the safe and efficient adoption of new technologies as they’re approved for clinical use. Bringing a new innovation from the completion of clinical trials to clinic adoption is very time and resource intensive, needing 10 years and $1 billion, on average.











