President’s Letter
2023 Metrics
Cycle of Translation
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
Jerold B. Katz Academy of Translational Research
Infectious Diseases Research Fund
Houston Methodist Dr. Mary and Ron Neal Cancer Center
Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist
The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative
Houston Methodist Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III
Translational Research Initiative
COVID-19 Studies
Outcomes Research
Restorative Medicine
Houston Methodist Advances Research into Neural Prosthetics
Noninvasive Spinal Stimulation Works to Restore Movement After Spinal Cord Injury
An Innovative Approach to Treat Even the Most Stubborn-to-Heal Fractures
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Houston Methodist, Rice University, Baylor College of Medicine Design Noninvasive Tech to Help Remove Brain’s Metabolic Waste
Houston Methodist Investigators Nanotechnology Investigators Awarded Prestigious Grants from the Department of Defense
Precision Medicine
Cancer Cell Type (Seed) and Tumor Microenvironment (Soil) Control Therapeutic Antibody Delivery and Efficacy
Novel Drug Combination Can Target Triple-Negative Breast Cancer for Treatment
A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine
Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease
New Virtual Intensive Care Unit Simultaneously Improves Patient Care and Bed Capacity
result
Introduction
Joint Weill Cornell–Houston Methodist Academic Institute Doctoral Program Welcomes its Inaugural Class
Visionary EnMed Program Soars to New Heights
Neural Control of Organ Degeneration and Regeneration (NeuralCODR) Training Program
Faculty and Research Development
Graduate Medical Education



Science in Service
of
Medicineresult
President's letter
2021 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

Ann Kimball and John W. Johnson Center for Cellular Therapeutics at Houston Methodist

Houston Methodist Dr. Mary and Ron Neal Cancer Center

The Food & Health Alliance within the Houston Methodist Lynda K. and David M. Underwood Center for Digestive Disorders, Immunology Center and the Fondren Inflammation Collaborative

Houston Methodist Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

Infectious Diseases Research Fund

From Discovery to Clinic


What is "Discovery to Clinic"?

Restorative Medicine


Houston Methodist Advances Research into Neural Prosthetics

Noninvasive Spinal Stimulation Works to Restore Movement After Spinal Cord Injury

An Innovative Approach to Treat Even the Most Stubborn-to-Heal Fractures

Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection

Houston Methodist, Rice University, Baylor College of Medicine Design Noninvasive Tech to Help Remove Brain’s Metabolic Waste

Houston Methodist Investigators Nanotechnology Investigators Awarded Prestigious Grants from the Department of Defense

Precision Medicine


Cancer Cell Type (Seed) and Tumor Microenvironment (Soil) Control Therapeutic Antibody Delivery and Efficacy

New Virtual Intensive Care Unit Simultaneously Improves Patient Care and Bed Capacity

Novel Drug Combination Can Target Triple-Negative Breast Cancer for Treatment

A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine

Importance of the Coronary Artery Calcium Score in Risk Assessment and Prevention of Atherosclerotic Cardiovascular Disease

Translational Luminaries




Discovery to Clinic

Precision Medicine
A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine
A Houston Methodist and Purdue University Breakthrough May Result in a More Effective Tuberculosis Vaccine
Despite the existence of the current tuberculosis (TB) vaccine, Mycobacterium tuberculosis (Mtb) remains a leading cause of mortality worldwide, accounting for approximately 1.5 million deaths each year. Multidrug-resistant TB (MDR-TB) is now compounding the significant public health and health security risks of TB. Chinnaswamy Jagannath, PhD, an expert TB researcher in the Department of Pathology and Genomic Medicine at the Houston Methodist Research Institute, has dedicated his life’s work to producing a more effective TB vaccine.
Bacillus Calmette-Guérin (BCG) is the current TB vaccine, developed 100 years ago. Because this vaccine isn’t as effective as needed, it would cost $13 billion U.S. per year for the level of prevention, diagnosis, treatment and care needed to reduce the incidence of and deaths from TB and achieve the UN global target determined at the 2018 meeting on TB. Clearly, a more effective vaccine would exponentially reduce that cost.

Chinnaswamy Jagannath, PhD
Previously, Jagannath and his team at Houston Methodist demonstrated that, once delivered, the BCG vaccine’s antigen is sequestered inside of phagosomes in antigen-presenting cells. If the antigen is held within these phagosomes, the BCG-derived Ag85B-p25 epitope cannot be presented to CD4 T cells. This significantly reduces the effectiveness of the BCG vaccine in priming the immune system to fight a natural TB infection.
To overcome this challenge, Jagannath and his team — consisting of Arshad Khan, PhD, Vipul Singh, PhD, and Abhishek Mishra, PhD — focused on developing a vaccine that releases TB antigens from the phagosomes by inducing autophagy mediated degradation and recycling of organelles. Their work, published in Nature Medicine, 2009; NPJ Vaccines, 2019, provides compelling evidence that autophagy induction can boost BCG efficacy.
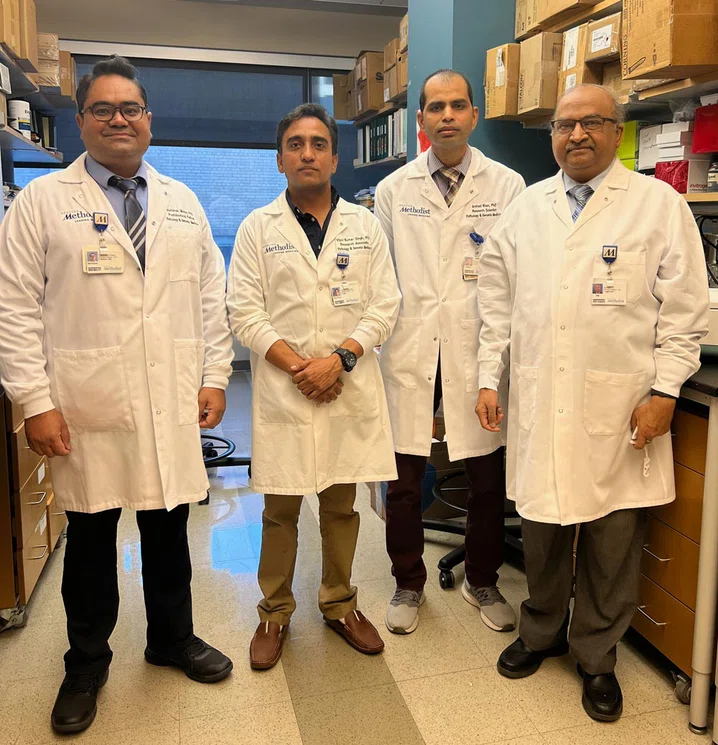
More recently, Jagannath and his colleagues discovered that Mtb itself contains a protein that harbors an autophagy-inducing peptide called C5. Building on that discovery, his team worked to develop an intranasal TB vaccine that included C5, although they needed an effective and safe vector to deliver the vaccine into cells. As a result, they collaborated with experts at Purdue University. Suresh Mittal, DVM, PhD, Distinguished Professor of Virology in Purdue's College of Veterinary Medicine, provided the intranasal bovine adenovirus vector critical for development of this groundbreaking vaccine candidate.
This collaboration resulted in a TB vaccine candidate containing the Mtb antigen Ag85B-p25 and C5 peptide in a replication-incompetent bovine adenovirus vector (BAdv85C5), a candidate that has shown significant success in murine studies. Jagannath and his team demonstrated that this strategy results in strong antigen presentation and excellent immune protection against TB, whether used as a booster after vaccination with BCG or as a single intranasal dose. Their article describing this notable work was published in the August 2021 issue of Cell Reports Medicine, and Jagannath and Mittal are the primary applicants on a patent for this vaccine platform. The research team is currently completing the preclinical studies and have submitted an NHP vaccine validation grant to the National Institutes of Health to test the vaccine, which will pave the way for human studies.
Developing a safe and effective vaccine is a marathon, not a sprint, and Jagannath made it clear he is in it for the duration when he said, “Children are tomorrow’s generation, so I’m working full time to get a TB vaccine developed that will protect them safely and effectively from TB.”
More from Discovery to Clinic