Science in Service
of
Medicine
President's Letter
Metrics 2019
Cycle of a Cure
Discovery to Clinic


Introduction

Precision Medicine


Introduction

Tracing Tau to Tackle Alzheimer's Disease

Translational Imaging Center Revamps for Revolutionary 7T MRI

Hope for Slowing ALS

The Heart of Progress: Innovative Valves Create a Legacy for the Future

Test-Driving Carbon Fiber Materials in Space

Lab-on-a-chip Shines Light on Bystander Effect

Creating an Antibody to Fight Silent Killers

A New View of Strep

Translational Luminaries
result



President’s letter
2019 Metrics
Cycle of a Cure
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
Building Blocks for Bone Regenration
RNA Therapeutics
Mobile App for Healthy Habits for Breast Cancer Survivors
Designing a Flexible Approach to Breast Reconstruction
Introduction
Tracing Tau to Tackle Alzheimer's Disease
Translational Imaging Center Revamps for Revolutionary 7T MRI
Hope for Slowing ALS
The Heart of Progress: Innovative Valves Create a Legacy for the Future
Test-Driving Carbon Fiber Materials in Space
Lab-on-a-chip Shines Light on Bystander Effect
Creating an Antibody to Fight Silent Killers
A New View of Strep
result

Tracing Tau to Tackle Alzheimer's Disease
Using multi-modal imaging and precision medicine to tackle dementia
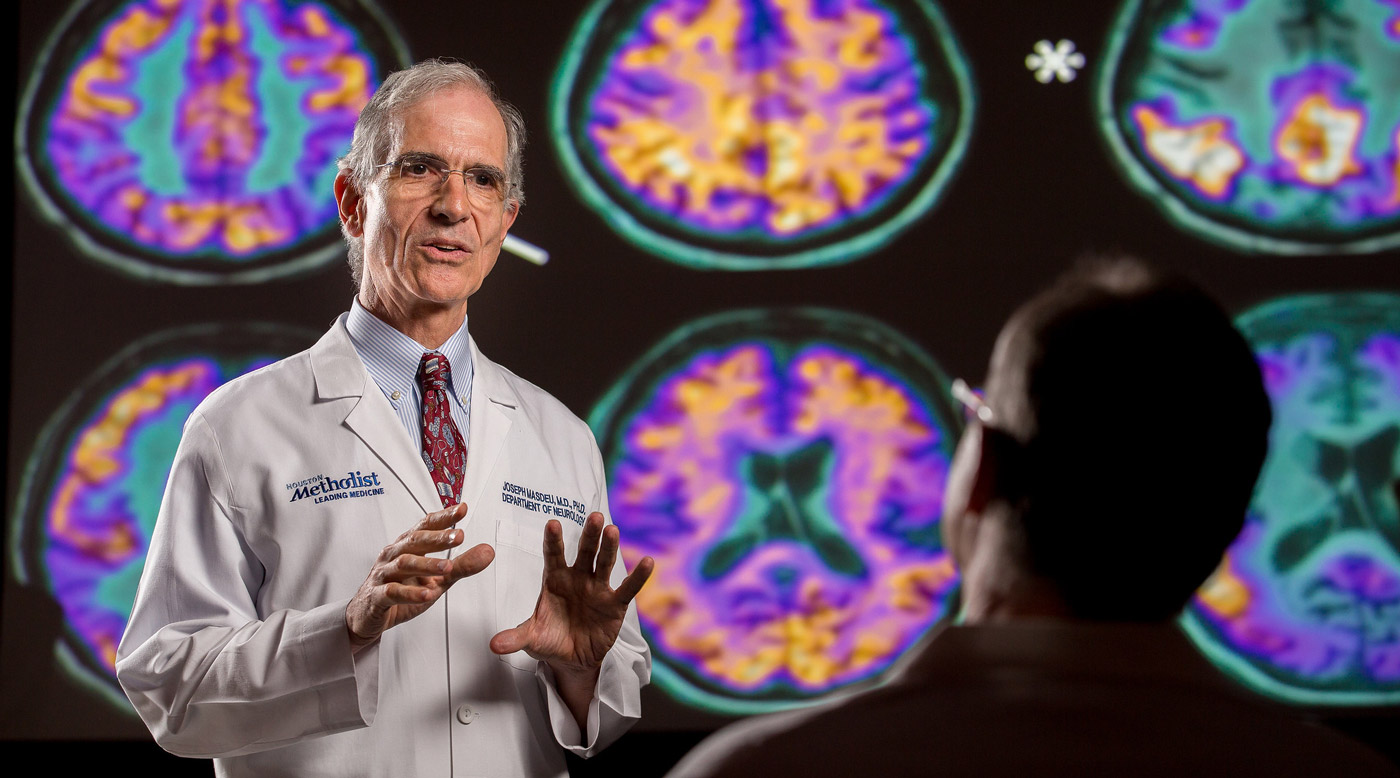
Joseph C. Masdeu, MD, PhD, studies the protein tau to understand how to prevent cognitive degeneration and tailor patient treatments based on unique personal profiles.
The Houston Alzheimer Study is a TMC-wide effort to define the abnormal chemistry leading to cognitive impairment, potentially earlier in life. By sharing data across institutions and connecting the dots using artificial intelligence, we will be empowered to understand how to prevent degeneration and tailor treatments to each patient’s unique personal profile.
—
Joseph C. Masdeu
, MD, PhDGraham Family Distinguished Chair for Neurological Sciences, Stanley H. Appel Department of Neurology
Director, Nantz National Alzheimer Center
Professor of Neurology
Houston Methodist
Houston Methodist neurologist Joseph Masdeu, MD, PhD, tends to think outside the box. And he applies this skill to what may be medicine’s greatest mystery: the brain and how it works.
Shortly after arriving at Houston Methodist in 2014, Masdeu took a bold step. He reported his findings that neurodegeneration in Alzheimer’s was associated with abnormal tau, a protein that forms inside cells from the normal tau that maintains the integrity of microtubules. At the time, most Alzheimer’s experimental therapies focused on the amyloid protein. But Masdeu did not find a similar association with the amyloid
protein, also found in this disorder.
In April 2019, Masdeu pushed forward with the concept that abnormal tau may spread in the brain through the normal brain highways, the white matter tracts that connect brain hubs to each other in a complex network arrangement. His study, published in
The Journal of Nuclear Medicine
, is the first to demonstrate this concept in vivo
in humans. Using sophisticated neuroimaging, Masdeu and his team, particularly Belen Pascual, PhD, combined MRI and 18F-flortaucipir PET to study tau in a rare disease that affects the language-related syntactic brain network. The anterior node of this network, in the frontal lobe, was affected first. Sick neurons found here, containing tau, contacted and "infected” neurons in the separate, but connected, posterior node in the temporal lobe. The white matter tract connecting both nodes was affected, as would be expected when the neurons in the anterior node died first.Pascual B, Funk Q, Zanotti-Fregonara P, Pal N, Rockers E, Yu MM, Spann B, Román GC,Schulz PE, Karmonik C, Appel SH, & Masdeu JC. Multimodal 18F-AV-1451 and MRI findings in non-fluent variant primary progressive aphasia: possible insights on nodal propagation of tau protein across the syntactic network. 2019,
Journal of Nuclear Medicine
, vol. 60, no. 7. This discovery reveals a window of opportunity for treatment, because to jump from neuron to neuron, tau appears to become extracellular
—
and possibly amenable to immunotherapy using antibodies that target this abnormal protein. Several pharmaceutical companies are interested in this approach: Three clinical trials are underway at the Nantz National Alzheimer Center of the Houston Methodist Stanley H. Appel Department of Neurology using anti-tau antibodies from Abbvie, Biogen and Eli Lilly. The Nantz National Alzheimer Center also is a leading partner in the Houston Alzheimer Study, a precision medicine cohort based in the Texas Medical Center, which intends to enroll 550 participants over age 40 to explore a range of cognitive disorders using advanced imaging, chemical analysis and artificial intelligence.
More from Discovery to Clinic
Contact Us
© 2021. Houston Methodist, Houston, TX. All rights reserved.
Privacy & Disclaimer
.