Science in Service
of
Medicine
President's Letter
Metrics 2019
Cycle of a Cure
Discovery to Clinic


Introduction

Precision Medicine


Introduction

Tracing Tau to Tackle Alzheimer's Disease

Translational Imaging Center Revamps for Revolutionary 7T MRI

Hope for Slowing ALS

The Heart of Progress: Innovative Valves Create a Legacy for the Future

Test-Driving Carbon Fiber Materials in Space

Lab-on-a-chip Shines Light on Bystander Effect

Creating an Antibody to Fight Silent Killers

A New View of Strep

Translational Luminaries
result



President’s letter
2019 Metrics
Cycle of a Cure
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
Building Blocks for Bone Regenration
RNA Therapeutics
Mobile App for Healthy Habits for Breast Cancer Survivors
Designing a Flexible Approach to Breast Reconstruction
Introduction
Tracing Tau to Tackle Alzheimer's Disease
Translational Imaging Center Revamps for Revolutionary 7T MRI
Hope for Slowing ALS
The Heart of Progress: Innovative Valves Create a Legacy for the Future
Test-Driving Carbon Fiber Materials in Space
Lab-on-a-chip Shines Light on Bystander Effect
Creating an Antibody to Fight Silent Killers
A New View of Strep
result

Cycle of a Cure
A Transformational Vision for Medical Research
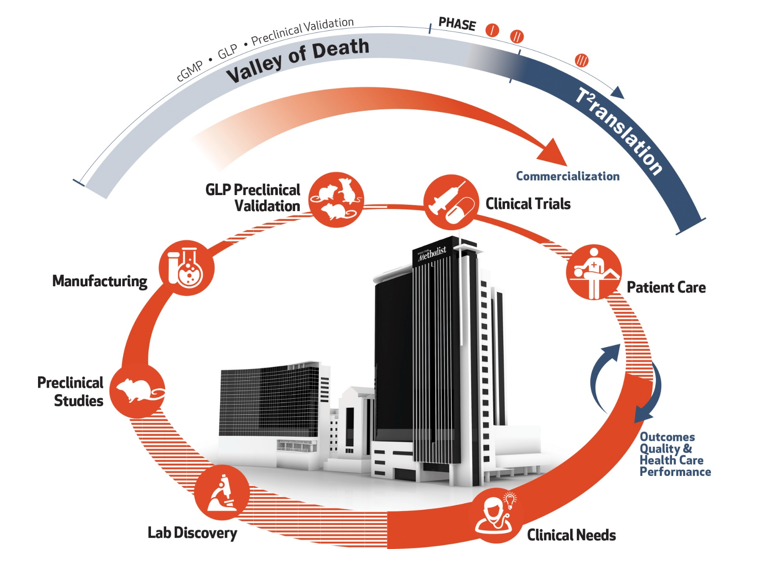
Houston Methodist met the challenge of translation early in the design of the research institute building. It houses the essential services and technology that support the full cycle of development to efficiently and effectively deliver innovations to the clinic.
Translating laboratory innovations into treatments for patients is fraught with challenges. The lack of specialized translational research resources makes it extremely difficult and expensive for most institutions to turn fundamental discoveries into tangible solutions that benefit the public. Their most promising innovations perish in the 'Valley of Death" before they reach the clinic. We provide support at every step of the cycle of a cure from bridge funding to technical expertise with U.S. Food and Drug Administration (FDA)-approved current good manufacturing practice (cGMP) facilities, good laboratory practice (GLP) facilities and clinical trial operations. T2 translation allows for the testing of new interventions in a controlled environment and allots time to gain knowledge about the ability of an intervention in an optimal setting. The most promising innovations have a lifeline at Houston Methodist.
Pinch to zoom image
Hover over the icons to learn more

Clinical trials
Teams have access to early phase trial support (pharmacokinetics and pharmacodynamics), and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials. This is the end phase of the cycle of a cure, opening the door to the future. Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.Discovery
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process. Interdisciplinary teams turn ideas into real solutions with sound testing and iterative design.Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.Current Good Manufacturing Practice (cGMP) Manufacturing
Houston Methodist is equipped with FDA cGMP-compliant facilities that produce research and clinical-grade therapeutic materials and custom radiopharmaceuticals for preclinical and first-in-human studies. Good Laboratory Practice (GLP) Preclinical Validation
The GLP facilities at Houston Methodist perform risk, safety and efficacy assessment studies in compliance with current FDA guidelines in preclinical models. Adherence to GLP standards is required for safety studies in order to move to clinical trials.Patient Care
The cycle begins and ends with patient care. Clinicians in our hospitals care for more than 1.5 million patients annually, enabling them to identify the most pressing challenges in medicine.
Patient Care
The cycle begins and ends with patient care. Clinicians in our hospitals care for more than 1.5 million patients annually, enabling them to identify the most pressing challenges in medicine.

Clinical Needs
Clinicians and researchers form interdisciplinary teams to address their needs and clinical challenges.

Discovery
Our teams of clinicians, researchers, and collaborators from around the world have access to a full suite of technology to enable the discovery process. Interdisciplinary teams turn ideas into real solutions with sound testing and iterative design.

Preclinical Validation
Research teams have access to dedicated Translational Research Initiative bridge funds to take the most promising discoveries into preclinical and early phase clinical development.

Current Good Manufacturing Practice (cGMP) Manufacturing
Houston Methodist is equipped with FDA cGMP-compliant facilities that produce research and clinical-grade therapeutic materials and custom radiopharmaceuticals for preclinical and first-in-human studies.

Good Laboratory Practice (GLP) Preclinical Validation
The GLP facilities at Houston Methodist perform risk, safety and efficacy assessment studies in compliance with current FDA guidelines in preclinical models. Adherence to GLP standards is required for safety studies in order to move to clinical trials.

Clinical Trials
Teams have access to early phase trial support (pharmacokinetics and pharmacodynamics), and outpatient clinical care and study management services, including research, nursing, regulatory submissions and budget management support for all phases of clinical trials. This is the end phase of the cycle of a cure, opening the door to the future.
Contact Us
© 2021. Houston Methodist, Houston, TX. All rights reserved.
Privacy & Disclaimer
.








