
President’s letter
2020 Metrics
Cycle of Translation
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics
The Fondren Food & Health Alliance and The Fondren Inflammation Center
Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III Translational Research Initiative
Jerold B. Katz Academy of Translational Research
result
Outcomes Research
Precision Medicine
CPRIT Funding to Drive New Discoveries in Cancer Therapeutics
Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries
Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers
Houston Methodist Institute for Technology, Innovation & Education (MITIESM)
Can Devices Provide A New Treatment Option for Glioblastoma?
Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available
Neuroimaging Offers New Insights into Neurodegeneration
COVID-19 Studies
Restorative Medicine
Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces
Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted
Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Revolutionizing the Future of Complex Valve Disease Management



Science in Service
of
Medicineresult
President's letter
2020 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics

The Fondren Food & Health Alliance and The Fondren Inflammation Center

Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

From Discovery to Clinic


Introduction

Restorative Medicine


Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces

Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted

Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones

Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection

Revolutionizing the Future of Complex Valve Disease Management

Precision Medicine


CPRIT Funding to Drive New Discoveries in Cancer Therapeutics


An Innovative New Tool to Enable Drug Discovery and Personalized Medicine


Devising a Novel Combination Treatment for Aggressive Double-hit Lymphoma



Expanding the RNAcore to Encompass the Entire Cycle of a Cure


Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries

Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers

Houston Methodist Institute for Technology, Innovation & Education (MITIESM)


Surgical Technology Developed in MITIE Gains FDA Approval


Pushing the Frontier of the Robotics Revolution

Can Devices Provide A New Treatment Option for Glioblastoma?

Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available

Neuroimaging Offers New Insights into Neurodegeneration

Translational Luminaries




Discovery to Clinic

Restorative Medicine
Revolutionizing the Future of Complex Valve Disease Management
Revolutionizing the Future of Complex Valve Disease Management
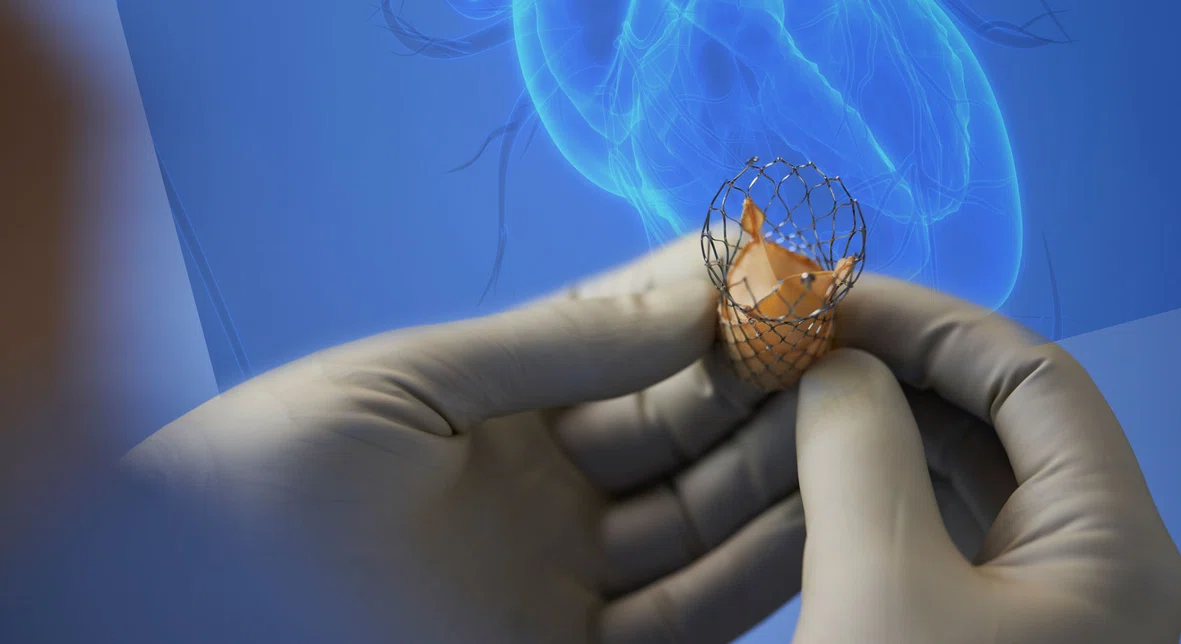
Replacing a faulty aortic valve once required open-heart surgery, which can pose significant risks for patients who are older or have certain comorbidities. The advent of minimally invasive transcatheter aortic valve replacement, or TAVR, procedure, where the replacement valve is inserted via a small incision in the groin or chest, has opened much needed treatment options for these patients.
“Momentous breakthroughs don’t happen often in medicine, TAVR is certainly one of the most exciting developments in recent times,” said Michael J. Reardon, MD, Allison Family Distinguished Chair of Cardiovascular Research in the department of cardiovascular surgery. “The Houston Methodist DeBakey Heart & Vascular Center’s valve clinic team has been at the very forefront of this pioneering innovation in minimally invasive surgery, making Houston Methodist one of the most experienced TAVR sites in the nation.” Under the leadership of Reardon and his cardiology partner Lois and Carl Davis Centennial Chair, Neal Kleiman, MD, the TAVR program has contributed to hundreds of publications in recent years and has played a key role in shaping this new field.
“TAVR is a force of positive disruption that is completely redefining the scope of valve replacement surgery. With the expanded indication, TAVR will be a game-changer for low-risk patients who are seeking to avoid the risks and longer recovery associated with surgery.”
– Michael J. Reardon, MD
Allison Family Distinguished Chair of Cardiovascular Research
Department of Cardiovascular Surgery
Houston Methodist
TAVR was initially approved by the FDA for use in patients with an intermediate or high risk of death or major complications during open-heart surgery. In August 2019, the FDA expanded approval to low-risk patients based on outcomes from two landmark clinical trials, Reardon was the national principal investigator for one of the trials. It confirmed that TAVR was a safe and effective treatment option for low-risk patients and was associated with shorter hospital stays, improved quality-of-life scores and a significantly lower rate of all-cause mortality or disabling stroke compared to traditional surgery. The complete findings are published in The New England Journal of Medicine.
More from Discovery to Clinic








