
President’s letter
2020 Metrics
Cycle of Translation
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics
The Fondren Food & Health Alliance and The Fondren Inflammation Center
Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III Translational Research Initiative
Jerold B. Katz Academy of Translational Research
result
Outcomes Research
Precision Medicine
CPRIT Funding to Drive New Discoveries in Cancer Therapeutics
Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries
Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers
Houston Methodist Institute for Technology, Innovation & Education (MITIESM)
Can Devices Provide A New Treatment Option for Glioblastoma?
Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available
Neuroimaging Offers New Insights into Neurodegeneration
COVID-19 Studies
Restorative Medicine
Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces
Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted
Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Revolutionizing the Future of Complex Valve Disease Management



Science in Service
of
Medicineresult
President's letter
2020 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics

The Fondren Food & Health Alliance and The Fondren Inflammation Center

Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

From Discovery to Clinic


Introduction

Restorative Medicine


Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces

Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted

Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones

Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection

Revolutionizing the Future of Complex Valve Disease Management

Precision Medicine


CPRIT Funding to Drive New Discoveries in Cancer Therapeutics


An Innovative New Tool to Enable Drug Discovery and Personalized Medicine


Devising a Novel Combination Treatment for Aggressive Double-hit Lymphoma



Expanding the RNAcore to Encompass the Entire Cycle of a Cure


Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries

Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers

Houston Methodist Institute for Technology, Innovation & Education (MITIESM)


Surgical Technology Developed in MITIE Gains FDA Approval


Pushing the Frontier of the Robotics Revolution

Can Devices Provide A New Treatment Option for Glioblastoma?

Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available

Neuroimaging Offers New Insights into Neurodegeneration

Translational Luminaries




Discovery to Clinic

Restorative Medicine
Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones
Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones

The Houston Methodist Center for Musculoskeletal Regeneration recognizes that the human body has an incredible capacity for healing and develops biomimetic implantables to augment these processes.

Francesca Taraballi, PhD

Bradley K. Weiner, MD
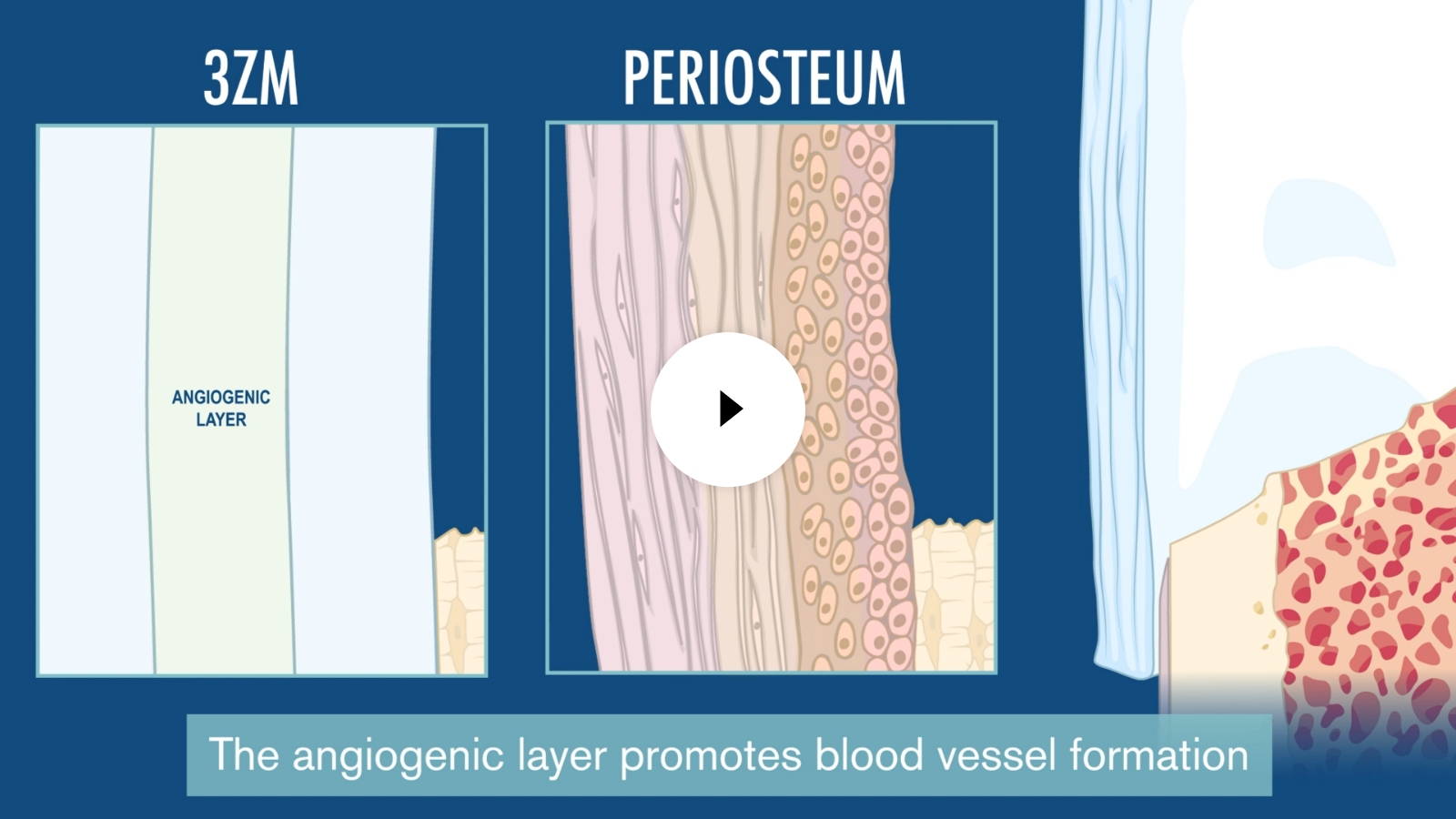
Center director Francesca Taraballi, PhD and her colleagues have demonstrated that all regenerative processes depend on a complex dialogue among different types of cells. They have developed a collagen-based scaffold device called 3ZM, which is currently in cGMP production, that enhances the structure and composition of growing bones to quickly repair complex fractures that would usually result in high infection rates, slow/incomplete healing or amputation.
In preclinical models, 3ZM guided immune, stem and bone membrane cells that remodeled the area into functional bone. Within six weeks, the fracture healed and the implanted materials were resorbed by the body, leaving the bone strong.
3ZM
Steps in Healing Process
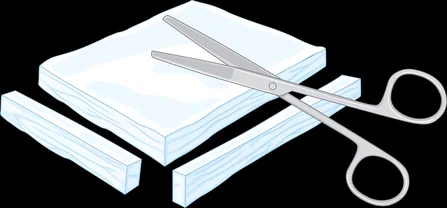
1

2
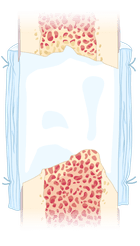
3
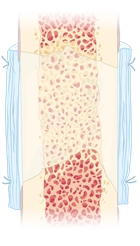
4


3 Zonal Membrane (3ZM) is cut and shaped to fit the defect site and then sutured to the surrounding periosteum. The process requires only one operation and no additional growth factors. After 4-6 weeks, the bone is fully healed, and the 3ZM is absorbed.
The Center was awarded $6 million by the United States Department of Defense under the leadership of Bradley Weiner, MD, professor of clinical orthopedic surgery, to complete preclinical cGMP assessment of 3ZM technology for a pre-Investigational Device Exemption regulatory consultation with the Food and Drug Administration. The Center leaders have worked with industry partners and regulatory experts and policymakers to identify an accelerated path to clinical application.
More from Discovery to Clinic








