
President’s letter
2020 Metrics
Cycle of Translation
Visionary Gifts

Discovery to Clinic

Innovative Education

Translational Luminaries
Introduction
The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics
The Fondren Food & Health Alliance and The Fondren Inflammation Center
Cockrell Center for Advanced Therapeutics
Paula and Joseph C. “Rusty” Walter III Translational Research Initiative
Jerold B. Katz Academy of Translational Research
result
Outcomes Research
Precision Medicine
CPRIT Funding to Drive New Discoveries in Cancer Therapeutics
Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries
Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers
Houston Methodist Institute for Technology, Innovation & Education (MITIESM)
Can Devices Provide A New Treatment Option for Glioblastoma?
Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available
Neuroimaging Offers New Insights into Neurodegeneration
COVID-19 Studies
Restorative Medicine
Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces
Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted
Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Revolutionizing the Future of Complex Valve Disease Management



Science in Service
of
Medicineresult
President's letter
2020 Metrics
Cycle of Translation
Visionary Gifts of Hope


Introduction

The Ann Kimbell and John W. Johnson Center for Cellular Therapeutics

The Fondren Food & Health Alliance and The Fondren Inflammation Center

Cockrell Center for Advanced Therapeutics

Paula and Joseph C. “Rusty” Walter III Translational Research Initiative

Jerold B. Katz Academy of Translational Research

From Discovery to Clinic


Introduction

Restorative Medicine


Houston Methodist and Rice University Launch Center for Translational Neural Prosthetics and Interfaces

Non-invasive Spinal Stimulation Enables Paralyzed People to Stand Unassisted

Dissolvable Implants Enhance the Body’s Ability to Heal Broken Bones

Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection

Revolutionizing the Future of Complex Valve Disease Management

Precision Medicine


CPRIT Funding to Drive New Discoveries in Cancer Therapeutics


An Innovative New Tool to Enable Drug Discovery and Personalized Medicine


Devising a Novel Combination Treatment for Aggressive Double-hit Lymphoma



Expanding the RNAcore to Encompass the Entire Cycle of a Cure


Siemens Healthineers and Houston Methodist Imaging Innovation Hub Empowers Researchers to Push the Boundaries

Novel Monoclonal Antibody Treatment Halts Tumor Growth in Deadly Ovarian and Pancreatic Cancers

Houston Methodist Institute for Technology, Innovation & Education (MITIESM)


Surgical Technology Developed in MITIE Gains FDA Approval


Pushing the Frontier of the Robotics Revolution

Can Devices Provide A New Treatment Option for Glioblastoma?

Houston Methodist Hospital’s new Paula and Joseph C. “Rusty” Walter III Tower offers the Most advanced treatments and innovations available

Neuroimaging Offers New Insights into Neurodegeneration

Translational Luminaries




Discovery to Clinic

Restorative Medicine
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Cell Encapsulation May Hold the Key to Preventing Cell Transplant Rejection
Cell transplantation is a promising option for the management of endocrine disorders such as type I diabetes and testosterone deficiency, but risk of rejection by the recipient’s immune system and the resulting life-long dependency on immunosuppressive drugs has been a major obstacle to long-term success.
In order to protect transplanted cells and improve cell viability, several research groups have tried encapsulating the cells within a capsule that acts as a physical barrier. However, issues with inadequate blood supply and oxygen permeability within the capsule have limited their ability to support the transplanted cells.

Alessandro Grattoni, PhD

A. Osama Gaber, MD
A Houston Methodist research team of nanomedicine and cell transplant experts led by Alessandro Grattoni, PhD, Frank J. and Jean Raymond Centennial Chair in the department of nanomedicine, has developed a novel encapsulation platform for transplanted cells termed neovascularized implantable cell homing and encapsulation or NICHE that directly addresses these limitations.

“Unlike other encapsulation platforms, NICHE successfully integrates blood supply, oxygenation and localized immunosuppressant drug delivery into one device. This has significantly improved cell viability, while avoiding the adverse effects of systemic immunosupression.”
Alessandro Grattoni, PhD
Chair, Department of Nanomedicine
Houston Methodist
NICHE is implanted just under the skin, permitting easy access for replenishing transplanted cells or drugs when needed. The researchers utilized 3-D printing technology to create the NICHE platform as it allows easy customization based on cell type and rapid, cost-effective scalability.
In a preclinical study published in Biomaterials, Grattoni and his team successfully used NICHE to transplant testosterone-producing testicular Leydig cells, offering a potential new treatment option for testosterone deficiency. The NICHE platform effectively established blood supply within the capsule and achieved sustained immunosuppressant delivery. Promisingly, the transplanted cells remained viable for the length of the study. Building upon their work with Leydig cells, the team is evaluating the efficacy of pancreatic islet cell transplantation using NICHE in preclinical diabetes models.

“These findings are a culmination of a long history of collaboration in cellular transplantation research at Houston Methodist and should expedite the translation of cell encapsulation technology from the lab to the clinic, delivering more effective treatment options to patients with conditions like type I diabetes.”
A. Osama Gaber, MD
John F. Jr. and Carolyn Bookout Presidential Distinguished Chair in Surgery, Department of Surgery
Houston Methodist

Credit: Biomaterials
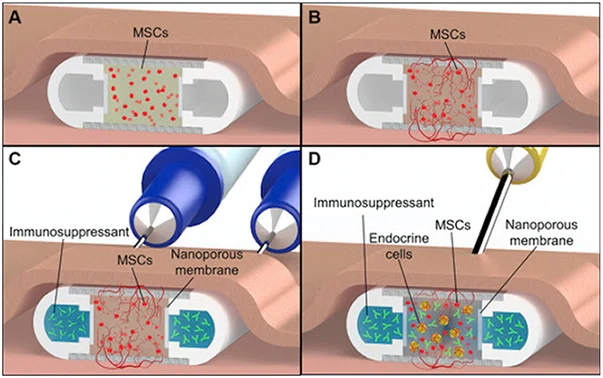
Fig. 1. NICHE deployment strategy. (A) Mesenchymal Stem Cells (MSCs) hydrogel-filled NICHE is implanted in a subcutaneous pocket to stimulate vascularization. (B) Pre-vascularization phase, with blood vessel formation across the cell reservoir. (C) Transcutaneous loading of immunosuppressant into NICHE drug reservoir using loading and venting needles. Needles are advanced into the NICHE drug reservoir through self-sealing silicon ports (visible in Fig. 2). Upon drug loading, the venting needle permits flushing of the reservoir and removal of entrapped air or liquid. (D) Transcutaneous transplantation of cells into the vascularized and immunosuppressed cell reservoir using a needle connected to a cell-loaded syringe.
More from Discovery to Clinic








