


Precision Medicine
Houston Methodist Team Carves a NICHE in State-of-the-Art Diabetes Therapy
Researchers Awarded R01 for Further Study of Subcutaneous Device for Management of Type 1 Diabetes
Researchers Awarded R01 for Further Study of Subcutaneous Device for Management of Type 1 Diabetes
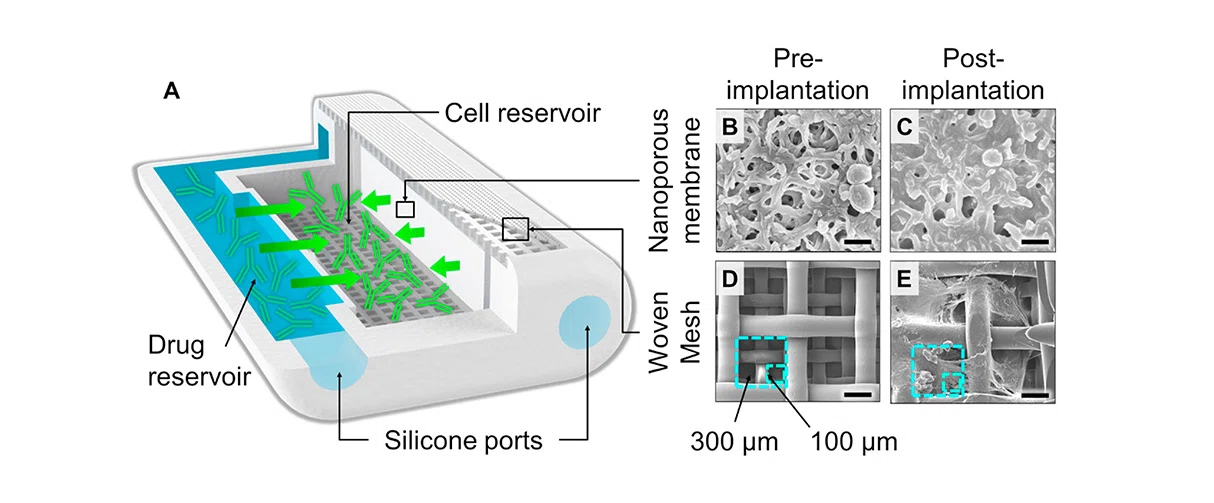
NICHE design. (A) Rendering of NICHE showing the cell and drug reservoir as well as the loading ports. SEM image of nylon nanoporous membrane before (B) and after (C) implantation. Scale bars represent 1 μm. SEM image of the two-layer nylon woven mesh before (D) and after (E) implantation. Scale bars represent 150 μm.
Image Credit: Neovascularized implantable cell homing encapsulation platform with tunable local immunosuppressant delivery for allogeneic cell transplantation. Biomaterials
Volume 257, October 2020.
Transplantation of healthy islets of beta cells from donors has long been viewed as a potential cure for type 1 diabetes. In islet transplantation, islets are taken from the pancreas of a deceased organ donor, purified, processed, and transferred into another person. Once implanted, the beta cells in these islets begin to make and release insulin. Usually, islets are infused into the portal vein of the liver for transplantation, but the risks involved include early loss of islets, bleeding or thromboses and the need for systemic immunosuppression. Other techniques are being investigated, such as injecting islets subcutaneously, which would be easier and, in theory, safer. Unfortunately, islet beta cells die even faster when subcutaneous, partly because they lack the ability to induce the vascular growth needed to provide the islets with oxygen and nutrients.
Design of the NICHE prioritizes clinical considerations of efficacy, safety and user acceptability. Cell and drug refilling of the NICHE reservoirs is transcutaneous and so can be done without removing the device, which allows for ease of drug replenishment when needed, thus extending implant lifespan potentially for the lifetime of patients.”
Alessandro Grattoni, PhD
Frank J. and Jean Raymond Centennial Chair, Chairman and Professor, Department of Nanomedicine.
At Houston Methodist, two researchers and their teams have been working together to address this issue. Alessandro Grattoni, PhD, a biomedical engineer with expertise in nanofluidics and implantable devices, and Joan Nichols, PhD, an immunologist with expertise in tissue engineering and transplant immunology, were awarded an NIH R01 this summer providing $2.8 million over a period of just under four years to continue studying their nanodevice called NICHE. NICHE is a subcutaneous vascularized encapsulation system designed to better facilitate islet cell transplantation for the treatment of type 1 diabetes. NICHE contains two reservoirs separated by a nanoporous membrane. One reservoir provides local immunosuppressant delivery that confines drugs to the graft site where immune attack occurs, minimizing exposure to the rest of the body and avoiding systemic immunosuppression and its associated adverse effects. The second reservoir - the cell reservoir - is fully vascularized with functional vessels, recreating an ideal physiological environment conducive to maintaining long-term viability and function of transplanted cells.
Alessandro Grattoni, PhD
Joan Nichols, PhD
Grattoni and Nichols have a long history of successful collaboration and this new R01 award represents the culmination of many smaller successes along the way. The first organization to financially support this work was the Vivian Smith Foundation, which has been continuously funding it for nine years at $100,00 per year. After the initial Vivian Smith funding, the work was awarded funding by the Juvenile Diabetes Research Foundation (JDRF): $200,000 for a proof-of-concept device. Then, internal funding from a pilot program created by Dirk Sostman, MD, at Houston Methodist provided another $250,000 for two years and they were able to leverage that into two more JDRF awards. With this new R01, the project currently has a combined $7 million in funding that will facilitate more preclinical studies, including a grant to specifically look at NICHE in preclinical models.
The newly awarded NIH R01 will allow the team to study several aspects of the NICHE platform in a diabetic small animal preclinical model. They will investigate the ability of mesenchymal stem cells to induce vascularization within the NICHE as well as modulate the NICHE immune microenvironment to be conducive to islet transplantation. This will be followed by the biodistribution analysis of immunosuppressants locally eluted in the NICHE and the assessment of immunomodulation and pharmacokinetics. Then they will evaluate efficacy of the NICHE in providing a suitable environment for successful islet engraftment to restore euglycemia in their model, assessed over the course of one year, in parallel to a longitudinal study of NICHE microenvironment remodeling.
Perhaps most importantly, design of the NICHE prioritizes clinical considerations of efficacy, safety and user acceptability. Cell and drug refilling of the NICHE reservoirs is transcutaneous and so can be done without removing the device, which allows for ease of drug replenishment when needed, thus extending implant lifespan potentially for the lifetime of patients. Further, the thin and compact size of the NICHE, which is smaller than the encapsulation implants under clinical investigation, is favorable for user acceptability. Successful completion of the proposed work will provide a broadly applicable encapsulation system with localized immunosuppressant delivery for long-term protection of transplanted islets, while minimizing adverse effects associated with immunosuppressive drugs. This could translate to a clinical breakthrough for deployment of cell therapies beyond islets, including stem cell-derived β cells, to treat diabetes as well as other diseases.
In addition to Grattoni and Nichols, key Houston Methodist personnel on the project include Ahmed Gaber, MD, Xian Li, MD, PhD, and Zhihui Wang, PhD.
Heather Lander, PhD
November 2022
Related Articles





