


Precision medicine
Houston Methodist Researchers Generate and Validate Humanized Biomimetic Nanovesicles to Target Neurons – Two Firsts in Nanovesicle Theranostics
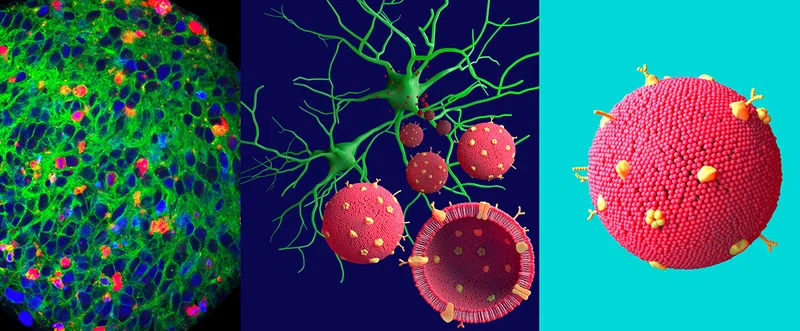
Restoring neuronal function after degeneration, traumatic injury or neuroinflammation can make the difference between a chronic injury or disability and full recovery. Efforts in this field have been impeded by the inability to precisely target neural cells with therapeutics or diagnostics. Precision targeting of specific neurons could also alleviate off-target effects of these cargos, which would allow treatment or diagnosis without altering the brain microenvironment – a critical aspect of safe and effective neuronal disorder treatments.
At Houston Methodist, researchers Francesca Taraballi, PhD, and Robert C. Krencik, PhD, are leading teams in a collaboration aimed at overcoming this obstacle to the treatment of brain disorders. Taraballi’s team is focused on “functionalizing” lipid based nanovesicles (NV) to target specific neuronal cell types. NVs are increasingly recognized as promising tools for delivery of diagnostics and therapeutics. Data indicates that surface engineering of NVs with membrane proteins from specific cell types increases NV biocompatibility and facilitates incorporation of functional attributes. This has been shown to work in other cell types, but Taraballi’s team is the first to try this approach with human neural cells for use within the nervous system. In order to get the nanovesicles “functionalized,” she needs Krencik’s team.
Krencik has a novel, well-defined and scalable approach to generating human neural cells from skin-sourced induced pluripotent stem cells. He then takes membrane proteins from the lab-derived human neurons and places those human proteins onto the nanovesicles, thus humanizing them. Through those human membrane proteins, the NVs can interact more seamlessly with the neurons being targeted for treatment. This then increases the ability of the nanovesicles to deliver cargo into those neurons. Using Krencik’s approach, humanized lab-derived neural cells can be easily scaled up, facilitating increased efficiency compared to using non-human animal models and taking the uniqueness of human-specific cells into account
We added neuron-derived proteins to lipid nanovesicles to functionalize them – so they interact better with neurons through those proteins. By improving the nanovesicle interactions with neurons, we can increase their ability to deliver things, like therapeutics, into those neurons.
Robert C. Krencik, PhD
Assistant Professor of Neurosurgery
In a paper recently published in Advanced Science, the combined teams optimized and validated the scalable and reproducible production of two kinds of neuron-targeting NVs, each having a distinct lipid formulation designed for potential therapeutic cargo, by integrating the human neuronal membrane proteins. Their results demonstrate that both genetically engineered and neuron-derived proteins are effectively incorporated into NVs without disruption of their physical characteristics. The humanized NVs with neuron-derived membrane proteins showed improved neuronal association and uptake compared to bare NVs.
A second critical and novel aspect of this study is to test the NVs efficacy on three-dimensional neural organoids. These neural organoids exhibit characteristics more similar to human brain as compared to traditional cell culture methods and the Krencik lab has pioneered methods to rapidly produce organoids that exhibit brain wave-like network activity. For this reason they can be used as high throughput screening platform to test the efficacy of different therapeutics upon neuronal functional repair. Here, they show that NV treatment does not disrupt viability of the neural organoids, which validates utility of organoid-based approaches as NV testing platforms. In addition, the team confirmed the results - cellular association and uptake of the biomimetic humanized NVs to neurons - within a murine cranial nerve model.
The customizable NVs reported in this study will facilitate design of functionalized diagnostics and therapeutics designed for neuroregeneration applicable to any disease in which neuronal regeneration is key to recovery, from traumatic brain injury and glioblastoma to stroke
Assaf Zinger, corresponding author 2 , Caroline Cvetkovic, Manuela Sushnitha, Tomoyuki Naoi, Gherardo Baudo, Morgan Anderson, Arya Shetty, Nupur Basu, Jennifer Covello, Ennio Tasciotti, Moran Amit, Tongxin Xie, Francesca Taraballi, corresponding author 1 and Robert Krencik corresponding author 3
Heather Lander, PhD, December 2021
Related Articles
Precision Medicine
The power of nanotechnology to manipulate theranostics and technology in cardiovascular disease management
Nanotechnology offers a steadily climbing number of applications and advances in the treatment and prevention of cardiovascular diseases - the leading cause of death in the United States.
PRECISION MEDICINE
New theranostic shows promise for treating triple-negative breast cancer
The radiolabeled 64Cu/177Lu-DOTA-diZD small synthetic molecule revolutionizes treatment strategies for primary and metastatic tumors in aggressive triple-negative breast cancer by targeting angiogenic receptors.






