Precision medicine
Variants, Vaccines and Vigilance
Variants, Vaccines and Vigilance
An updated look at circulating SARS-CoV-2 variants, with a dose of more effective vaccine booster scheduling, tailored by Houston Methodist researchers applying Mathematics in Medicine.
Information in this article was accurate at the time of publication.
For the latest information visit the Centers for Disease Control & Prevention (CDC), World Health Organization (WHO), and your state and local government web pages.
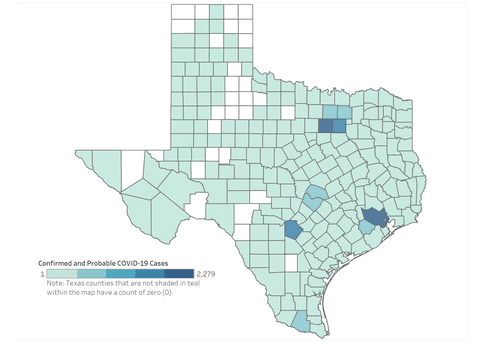
Since July 2023, the number of positive COVID tests and hospitalized covid patients has been increasing across the U.S. During the first 19 days of 2024, in Texas, there were more than 38,000 (confirmed and probable) reported cases of COVID-19. When the CDC said the pandemic was over last year, what they meant was that SARS-CoV-2 was endemic, not that it no longer posed a risk.
Current Variants of Interest
In the past year, there has been numerous emerging SARS-CoV-2 variants (an inevitability considering the speed of reproduction and rate of mutations in any circulating virus), only a handful were considered worth following. These usually exhibited significant changes in their spike protein, which could have led to increased transmission or virulence. So far, existing immunity from vaccines and previous infections still offers some level of protection.
Last summer, variant BA.2.86—nicknamed Pirola—emerged. A descendent of Omicron, BA.2.86 contained a significant number of spike protein mutations. In fact, the scope of evolutionary jump seen in BA.2.86 relative to Omicron was likened to that between Omicron and the original SARS-CoV-2 strain, so experts watched it closely. As fall slid into winter, BA.2.86 remained epidemiologically unexceptional, though it gave rise to the variant currently taking over—JN.1.
JN.1 contains merely one mutation in the spike protein relative to BA.2.86. Yet, as of March 26, 2024, it accounts for ~96% of all circulating SARS-CoV-2 variants, more than double its prevalence at Christmas. Currently, there is no evidence that JN.1 causes more severe disease though preliminary research shows that it may exhibit enhanced immune evasion.
“Even if it is more evasive, at this point most everyone has some level of immunity and some level of memory cells to SARS-CoV-2,” said Wesley Long, MD, PhD, Associate Professor of Pathology and Genomic Medicine and Director of the Diagnostic Microbiology Laboratory. “That may mean you’d be more likely to get a symptomatic infection than not.”
And now there is a new variant on our radar—BA.2.87.1—that has been detected in nine samples from South Africa. BA.2.87.1 is interesting and possibly concerning because it bears over 30 spike protein mutations relative to XBB.1.5. XBB.1.5 is the variant against which the 2023-2024 SARS-CoV-2 vaccine was designed. For that reason, the number of mutations could be relevant to vaccine efficacy. However, the samples from which this variant was identified were collected between September and December 2023, with results reported on January 31, 2024. This means BA.2.87.1 has been circulating since September 2023 without any detectable increase in prevalence and no apparent epidemiological impact.
Nonetheless, the CDC is tracking BA.2.87.1 and as of February 8, 2023, there have been no cases of BA.2.87.1 identified anywhere beyond South Africa.
While we don’t yet have data on vaccine/existing immunity efficacy against BA.2.87.1, it’s likely that the trend we’ve seen so far will hold—that existing immunity either from vaccines or previous infections will confer some degree of protection against severe disease and death.
Vaccine
Dosing Schedule
The current available vaccines against SARS-CoV-2 are safe and effective, but waning immunity in the population and accumulation of mutations in the virus mean we need booster doses to stay protected.
As it stands, boosters come in the form of updated vaccines. When a new version of the vaccine is generated against emerging variants, it is recommended we get the updated vaccine. Current CDC recommendations are to get the updated vaccine at least eight weeks after your last dose. If you’ve recently had a natural infection with SARS-CoV-2, they recommend delaying that updated dose by three months.
Is that the best booster dosing schedule for everyone? Houston Methodist researchers asked this very question. In a research project led by Prashant Dogra, PhD, Assistant Research Professor, Mathematics in Medicine Program, aimed to optimize vaccine dosing schedules to ensure prolonged continuity in protection.
Prashant Dogra, PhD
Toward this end, his team developed a mechanistic model of immune response to vaccines as a tool for dosing schedule optimization. Clinical data sets of acquired immunity to COVID-19 mRNA vaccines in healthy and immunocompromised participants were used to calibrate the model, which then accurately predicted neutralizing antibody kinetics in response to multiple doses of COVID-19 mRNA vaccines. Perhaps most impressive is that the model allowed them to calculate tailored vaccination dosing schedules by estimating population vulnerability to breakthrough infections. They show that small modifications in the CDC recommended dosing scheduling may increase protection in individuals with varying levels of compromised immunity.
Model-predicted optimal dosing and CDC-recommended dosing schedules for the Pfizer-BioNTech vaccine in healthy and immunocompromised populations. The ongoing CDC guidelines for dosing schedules are represented by the blue bands, and those recommended by the model are shown in green.
For example, in patients with significant immunosuppression, delaying the second dose only slightly results in increased antibody production and greater protection. A result likely due to the need for immune recovery in immunocompromised individuals after the first dose.
The study, published in 2023 in JCI Insight, covers the immunocompromised, but the research team is also applying the model to other subpopulations, such as pregnant women, pediatrics, racial and ethnic groups and the elderly.
“Forecasting immune response allows us to identify dosing strategies that may improve vaccine effectiveness and ensure long-term protection against the virus, based on the needs of individual subpopulations,” said the study’s corresponding author Dogra.
Their work also has implications for vaccine distribution and compliance. Given the disparities in global vaccine allocation, optimization of dosing schedules to extend the gaps between doses without compromising efficacy could allow for improved vaccine distribution to countries with limited resources and promote vaccine compliance.
By applying their model to different infectious diseases and vaccines, the team will add personalized medicine to the pandemic toolbox that can help guide more effective public health policies as well as inform development of more effective vaccines.
Stay
Vigilant
Now is not the time for complacency. The COVID-19 surge that peaked at the turn of the new year was the second-highest surge of infections of the pandemic. While recent illness caused by COVID-19 has been mostly mild, it is still a major cause of serious respiratory disease that can lead to hospitalization and death. Additionally, infection can have long-term consequences, which can be cumulative and even healthy people can develop Long COVID—when symptoms (often debilitating) last four or more weeks after initial infection.
May 2024
Related Articles