


Precision Medicine
A Novel and Potent Tumor Cell Autosis Technique
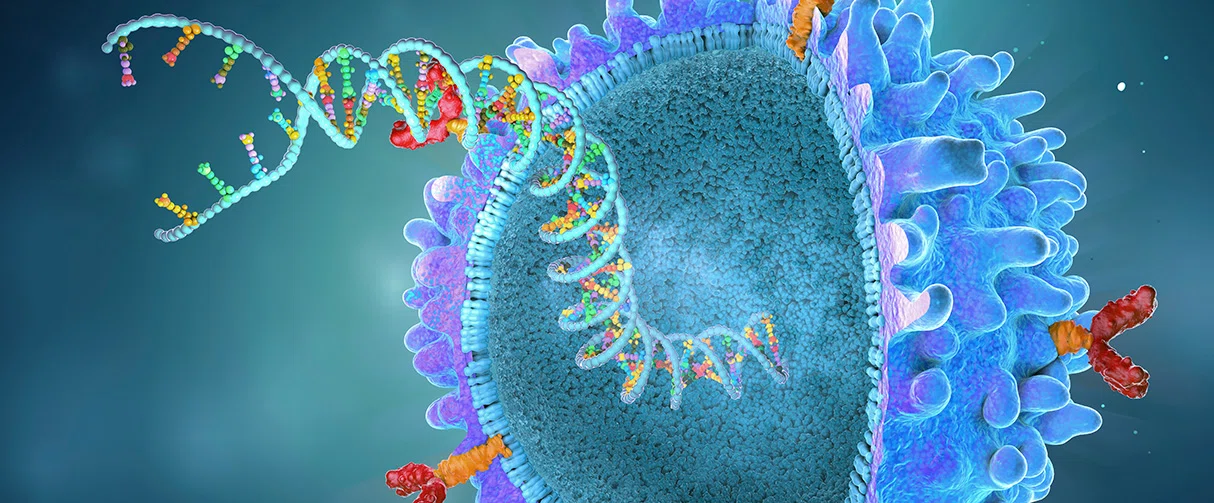
A novel and potent tumor cell autosis technique has been brought to Houston Methodist by Yong Lu, associate professor of Immunology, that can enhance the effectiveness of cancer immunotherapies. Lu joined Houston Methodist in November 2021, and his research focuses on adoptive cell therapies, including chimeric antigen receptor (CAR) T-cell therapy.
The downside of classic CAR T-cell therapy is an insufficient primary response, which leads to the inefficacy of this treatment. To increase the killing of tumor cells, Lu designed an alternate CAR T-cell therapy technique using T cells infected with the myxoma virus (MYXV). The MYXV is a naturally occurring oncolytic virus (a member of the Leporipoxvirus genus) that is safe for humans and all non-leporid animals. It only infects rabbits, hares and leporids in nature.

Yong Lu, PhD
Associate Professor of Immunology
at Houston Methodist
This double-stranded DNA virus was used in the 1950s to control the European rabbit population in Australia. In the South American tapeti (its natural host), the MYXV causes an innocuous, localized cutaneous fibroma. However, in European rabbits, the MYXV leads to a fatal disease called myxomatosis, with a greater than 90% mortality rate. Myxomatosis is characterized by tumor formations in various parts of the body and symptoms, including swollen head, eyelids and ears, and raised cutaneous lesions over the body, legs and ears. The molecular and genetic basis of the lethality of the MYXV in European rabbits (in stark contrast to non-lethality in other animals) is not understood.
Oncolytic virotherapy (OV) is a relatively new and promising therapeutic strategy for malignant cancers. Currently, several therapies and treatment strategies exist for cancer management such as surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, hormone therapy, stem cell therapy and CAR T-cell therapy. OV can be used as a monotherapy as well as in combination with other therapies.
It offers several advantages including utility against a wide variety of cancers, selective killing of tumor cells, activation and recruitment of immune cells to the tumor microenvironment and in some cases targeting cancer in metastatic sites. Furthermore, the anti-tumor activity of oncolytic viruses can be enhanced by genetic modifications. On the other hand, there are drawbacks to OV. In metastatic tumor beds, there is insufficient delivery of oncolytic viruses using standard intravenous infusions.
We developed a special strategy for systematically delivering the oncolytic myxoma virus into solid tumors by transferring tumor-specific T cells infected with the myxoma virus. Remarkably, eradication of solid tumors was associated with induction of tumor cell autosis, which is unlike the classic cytolytic machinery of T cell cytotoxicity and causes potent bystander killing of tumor cells. Our study uncovers an unexpected T cell-killing mechanism of tumor cells by T cells infected with the myxoma virus, which could catalyze a paradigm shift in adoptive cell therapy to overcome therapeutic resistance in solid tumors.
Yong Lu, PhD
Associate Professor of Immunology
at Houston Methodist
One unique function of oncolytic viruses, including the MYXV, is the selective infecting and killing of tumor cells. Normal cells cannot be infected or killed by the MYXV, and MYXV is safe for humans.
Once the CAR T cells infected with the MYXV are injected into the patient, the T cells locate the tumor via antigen recognition and release the virus into the tumor microenvironment. The advantage of using the MYXV-infected CAR T cells (as compared to CAR T cells alone) is the 100% kill rate of tumors. This strategy can be used to target all kinds of solid tumors including carcinomas, sarcomas and lymphomas. This is particularly significant since the majority of cancer deaths are caused by solid tumors.
According to Lu, “We developed a special strategy for systematically delivering the oncolytic myxoma virus into solid tumors by transferring tumor-specific T cells infected with the myxoma virus. Remarkably, eradication of solid tumors was associated with induction of tumor cell autosis, which is unlike the classic cytolytic machinery of T cell cytotoxicity and causes potent bystander killing of tumor cells. Our study uncovers an unexpected T cell-killing mechanism of tumor cells by T cells infected with the myxoma virus, which could catalyze a paradigm shift in adoptive cell therapy to overcome therapeutic resistance in solid tumors.”
Lu has currently filed a patent to protect this unique technique and is in the process of evaluating and optimizing other oncolytic viruses for similar strategies. Several oncolytic viruses have been assessed in clinical trials after successful results in preclinical models. In the near future, the MYXV-infected CAR T cells are expected to be similarly evaluated.
Ningbo Zheng, Jing Fang, Gang Xue, Ziyu Wang, Xiaoyin Li, Mengshi Zhou, Guangxu Jin, Masmudur M Rahman, Grant McFadden, Yong Lu. Induction of tumor cell autosis by myxoma virus-infected CAR T and TCR T cells to overcome primary and acquired resistance.Cancer Cell. 2022 Sep 12;40(9):973-985.e7.
The research work on the myxoma virus infected CAR T therapy by Dr. Yong Lu was supported by the National Cancer Institute (NCI; 1R37CA251318-01, 1R01CA248111-01A1, R01CA258477-01, and 1R01CA264102-01) and CPRIT Scholar (RR210067).
Abanti Chattopadhyay, PhD
April 2023
Related Articles





