clinical research
Renal Cell Carcinoma Treatment Landscape and Challenges
Renal Cell Carcinoma Treatment Landscape and Challenges
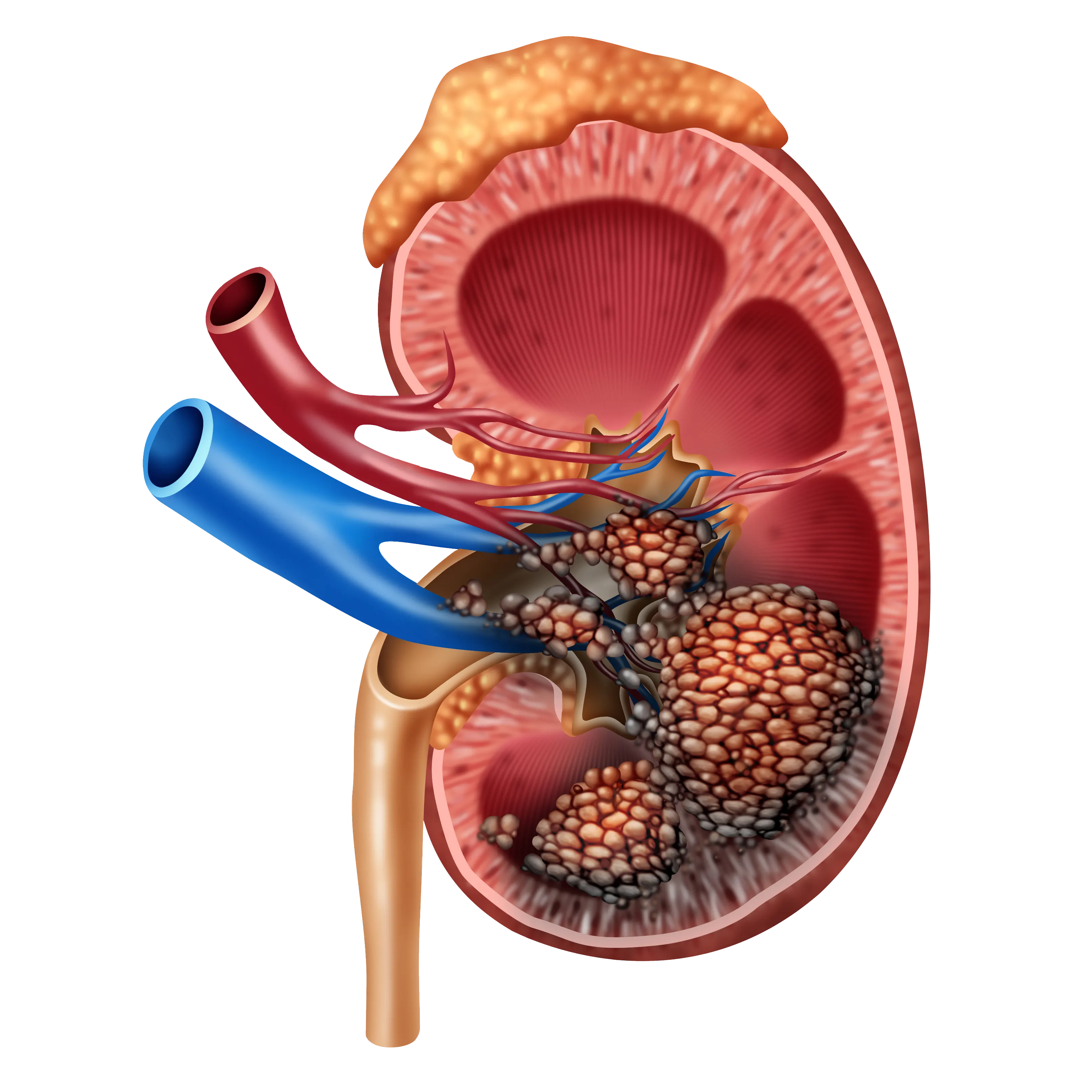
Kidney cancer is one of the top ten common cancers in both men and women. According to the World Cancer Research Fund International, there were more than 430,000 new cases of kidney cancer in 2020.
The five-year recurrence rate for high-risk localized RCC is an alarming 68%.
Renal cell carcinoma (RCC) is the most common type of kidney cancer that forms in the lining of the kidney tubules. Other types of kidney cancer include urothelial carcinoma, Wilms tumor — a rare cancer that mainly affects children, renal sarcoma and benign kidney tumors. The removal of the organ is the standard of care for localized RCC; however, the five-year recurrence rate for high-risk localized RCC is an alarming 68%.
Share this story
Raj Satkunasivam, MD
Raj Satkunasivam, MD, Associate Professor of Urology, performed a meta-analysis of four recent Phase III randomized controlled trials (RCTs) to evaluate the benefit and safety of immune checkpoint inhibitors (ICIs) as adjuvant therapy in RCC. Satkunasivam concluded that patients with positive programmed death-ligand 1 (PD-L1) expression or sarcomatoid features are most likely to benefit from adjuvant immunotherapy. The details of this review study were published in the British Journal of Urology International in 2023.
Because of the considerable heterogeneity between studies, mixed evidence exists to date regarding ICIs as adjuvant therapy for RCCs. Satkunasivam and his team searched the PubMed, Embase, and conference proceedings (inception until 2022) for the terms: “adjuvant,” “immunotherapy,” and “renal cell carcinoma.” The initial literature search yielded 828 unique records, which after screening, led to the identification of four RCTs with a total of 3,407 patients.
The primary endpoint was disease-free survival (DFS) and secondary endpoints included rates of serious adverse events (AEs), immune-related AEs and treatment discontinuation due to AEs. Quality assessments were performed to evaluate randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, attrition bias and selective reporting.
- KEYNOTE- 564 (using prembrolizumab; published in 2021 and 2022)
- PROSPER (using nivolumab; published in 2022)
- CheckMate 914 (using nivolumab and ipilimumab; published in 2022)
- IMmotion010 (using atezolizumab; published in 2022)
The four RCTs analyzed were:
The four RCTs analyzed were:
- KEYNOTE- 564 (using prembrolizumab; published in 2021 and 2022)
- PROSPER (using nivolumab; published in 2022)
- CheckMate 914 (using nivolumab and ipilimumab; published in 2022)
- IMmotion010 (using atezolizumab; published in 2022)
The American Cancer Society recently estimated that 14,980 people (9,920 men and 4,970 women) would die from the disease in 2023; the average age at which kidney cancer is diagnosed is between 64 and 74. Some of the biggest risk factors for kidney cancer include obesity, smoking, high blood pressure, hypertension and certain inherited syndromes, and race.
Satkunasivam observed substantial heterogeneity when pooling results from these RCTs for DFS with no significant benefit in overall DFS. The heterogeneity observed can be attributed to differences in drug/ICI target, drug tolerability, inclusion criteria, cohort composition, interventions and statistical considerations. To offset the inter-study heterogeneity, Satkunasivam used random effects models in the meta-analysis. Furthermore, sub-group analyses identified that populations with high PD-L1 expression or sarcomatoid features benefitted from ICI adjuvant therapy.
There are over a dozen sub-types of RCC that are differentiated by the microscopic appearance of the tumor. Clear cell RCC (ccRCC) is the most common sub-type in which the cancer cells appear pale and clear. The standard of care for metastatic ccRCC is ICI therapy either as doublets or in combination with tyrosine kinase inhibitors.
There is an immediate need to determine the overall benefit of adjuvant immune checkpoint inhibitors in renal cell carcinoma in light of multiple disparate trials. Meta-analyses aim to combine comparatively similar trials to increase the power to detect an effect. However, substantial heterogeneity makes the conclusions from summative statistics misleading. We believe our data will help frame the overall adjuvant immune checkpoint inhibitor landscape discussion while we await results from ongoing phase III trials. Additionally, we believe that early analyses of negative trials, although not peer-reviewed, will promote discussion and debate regarding the use of adjuvant immunotherapy in patients with renal cell carcinoma.
Raj Satkunasivam, MD
Associate Professor of Urology
Adequate patient selection is integral to adjuvant therapy in RCC. Presently, there is an unmet need for reliable biomarkers toward this end. Interestingly, adjuvant prembrolizumab only received a weak recommendation for therapy in patients with high-risk ccRCC despite being approved by the United States Food and Drug Administration.
“There is an immediate need to determine the overall benefit of adjuvant immune checkpoint inhibitors in renal cell carcinoma in light of multiple disparate trials,” said Satkunasivam. “Meta-analyses aim to combine comparatively similar trials to increase the power to detect an effect. However, substantial heterogeneity makes the conclusions from summative statistics misleading. We believe our data will help frame the overall adjuvant immune checkpoint inhibitor landscape discussion while we await results from ongoing phase III trials.
“Additionally, we believe that early analyses of negative trials, although not peer-reviewed, will promote discussion and debate regarding the use of adjuvant immunotherapy in patients with renal cell carcinoma.”
The RCC treatment landscape is evolving and further developments with new technologies and strategies will help physicians better predict drug efficacies and improve patient stratification.
Carlos Riveros, Emily Huang, Sanjana Ranganathan, Zachary Klaassen, Brian Rini, Christopher J D Wallis, Raj Satkunasivam. Adjuvant immunotherapy in renal cell carcinoma: a systematic review and meta-analysis. BJU Int. 2023 May;131(5):553-561. doi: 10.1111/bju.15981.
Abanti Chattopadhyay, PhD
March 2024