Clinical Research
Strides in Dialysis Access
First to perform minimally invasive surgery
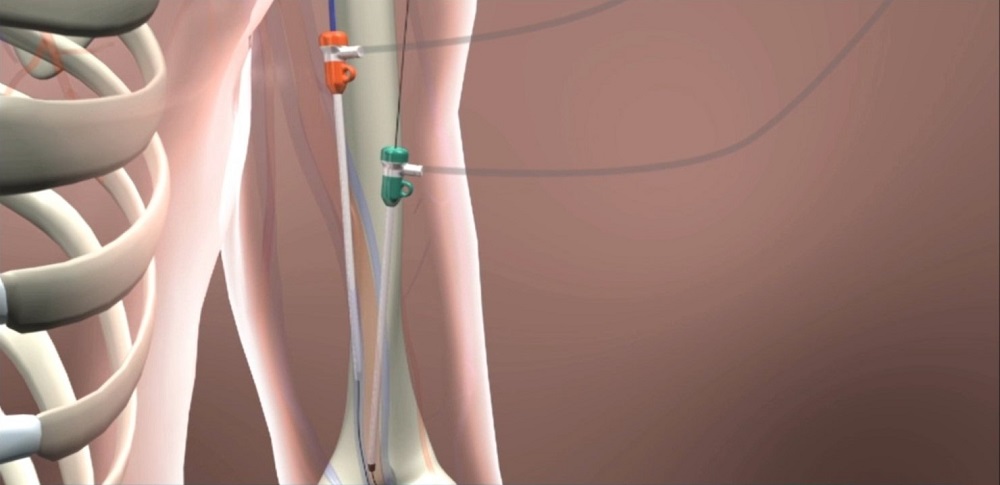
This new technique may lead to fewer complications and surgeries for the 470,000 Americans who undergo dialysis two to three times a week.
This new technique may mean fewer complications and surgeries for the 470,000 Americans who undergo dialysis two to three times a week.
On August 22, Eric K. Peden, MD, chief of vascular surgery at Houston Methodist DeBakey Heart & Vascular Center, became the first surgeon in the U.S. to perform minimally-invasive surgery to create dialysis access for patients with severe kidney disease.
Eric Peden, MD, was the first surgeon in the U.S. to perform minimally-invasive surgery for dialysis patients with severe kidney disease.
Peden made one small puncture in a patient’s artery and another in the vein, using a device called the WavelinQ™ endoAVF System by Becton Dickinson. It had two magnets attached on the end of a catheter to pull the artery and vein together and—using a radio frequency—created a hole between the two that allowed them to connect.
When the system was recently approved by the Food and Drug Administration, Houston Methodist was the clear choice to perform the first surgery. Parts of the new system had been introduced at one of the earliest Pumps & Pipes, a symposium that created a platform for cross-industry collaboration to solve challenging problems shared by all. Its organizers included ExxonMobil, NASA and Houston Methodist.
When the time came to test the device, several preclinical trials were conducted at the Houston Methodist Institute for Technology, Innovation & Education. Also known as MITIE™, it will become a site for training more surgeons in using the new procedure.
“One problem with open surgery is that suturing together the vein and artery sometimes creates an inflammatory response, which may cause the connection to fail to develop adequately for dialysis,” said Peden. “This new technique decreases the risk of this happening.”
This story reflects the life cycle of medical device development, from preclinical testing to clinical trial, FDA approval and finally physician training. We have built systems that allow us to participate in all phases of this process.

Alan B. Lumsden, MD
Walter W. Fondren III Distinguished Endowed Chair
DeBakey Heart & Vascular Center
Chair and Professor, Department of Cardiovascular Surgery
Houston Methodist
George Kovacik, August 2018