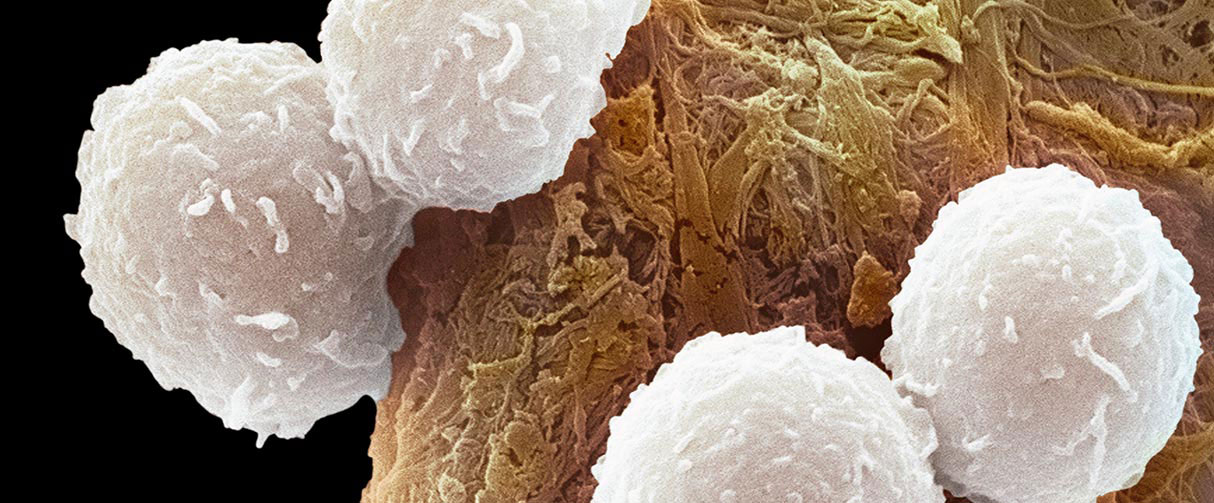
Resembling fuzzy snowballs under an electron microscope, CD8+ T immune cells are studded with a rich repertoire of proteins on their outer membrane that helps keep cancers and other diseases at bay. But when these immune cells are in the close vicinity of developing tumors, the aberrant microenvironment can trigger T cell death, thereby compromising the body’s cancer-fighting action.
Researchers from the Houston Methodist Dr. Mary and Ron Neal Cancer Center have now shown that fatty acids in tumor microenvironments set off an overexpression of the membrane protein CD36 in CD8+ T cells, which then programs the cells toward ferroptosis, a type of self-destructive cell death. However, they also found that blocking CD36 can restore the immune cells’ cancer-fighting action and even enhance it if paired with checkpoint inhibitors used in immunotherapy.
“Our goal is to find out the major players causing T cell dysfunction within the tumor microenvironment,” said Qing Yi, MD, PhD, professor of oncology and senior author on the study. “This knowledge will help design better immunotherapy interventions that can treat more patients and cancers in the future.”
The researchers have reported their findings in the journal Cell Metabolism.
Cancerous cells thrive within tiny ecosystems or microenvironments that contain blood vessels and other biological materials, including the extracellular matrix, fatty acids and signaling molecules. Hence, the interaction between the tumor and its environment plays a crucial part in cancer growth and progression. For example, when the nutrients supplied to the tumor through the bloodstream are low, fatty acids within the microenvironment facilitate the rapid growth of cancerous cells.

Qing Yi, MD, PhD
Ralph O’Connor Centennial Chair,
Dr. Mary and Ron Neal Cancer Center
Director, Center for Translational Research in Hematological Malignancies
Professor of Oncology
Houston Methodist
Immune cells are also part of the tumor microenvironment milieu. But despite their ability to combat cancers, many lines of evidence show that immune cells, particularly the CD8+ T cells, become mysteriously disarmed within the tumor microenvironment. The researchers explained that the mechanisms underlying the T cells’ loss of function near tumors could be a reason why the success of immunotherapies with CAR T cells and checkpoint inhibitors has been limited.
Yi’s team had previously shown that cholesterol within the tumor microenvironment is detrimental to CD8+ T cells’ cancer-fighting action. To investigate this phenomenon in more depth, the researchers focused on CD36, a scavenger receptor that plays an important role in lipid metabolism in a variety of immune cells.
First, the team studied the genetic profile of CD8+ T cells isolated from melanoma patients and B16 melanoma mice. In both human and murine CD8+ T cells, the level of CD36 expression scaled with tumor progression, and the presence of cholesterol in vitro induced more expression of CD36 in the T cells. The detailed investigation also revealed that the genes associated with lipid peroxides and ferroptosis activation were highly expressed, indicating that CD8+ T cells likely internalize fatty acids that are present in the tumor microenvironment via the CD36 receptors and then overtime accumulate enough lipid peroxides to trigger ferroptosis.
To test ways of restoring anti-tumor action, the researchers treated rodent CD8+ T cells with a ferroptosis inhibitor, ferrotstatin-1. When these cells were injected into melanoma mouse models, they found that the anti-tumor action was enhanced. Next, they engineered CD8+ T cells lacking CD36 receptors and then injected these cells along with antibodies for checkpoint inhibitor PD-1 into melanoma-bearing mice. They found that the level of cytokines, a signature of anti-tumor activity, was significantly more elevated in these mice compared to those that were treated with just PD-1 or wild-type CD8+ cells.
Infographic is designed by Sara Carr
The researchers noted that while their work presents evidence of how CD36 could cause CD8+ T cells to lose their function near tumors, the exact biochemical pathways leading up to ferroptosis remain to be unraveled. Further, CD36-triggered ferroptosis may not be the sole mechanism compromising CD8+ T cell function in the tumor microenvironment.
Despite these limitations, they said, their findings could potentially lead to treatments that boost the efficacy of T cell-based immunotherapy.
“Unless we know which branch of the mechanism plays the most important role in T cell dysfunction, we can’t yet design a single remedy or treatment strategy to overcome the problem,” said Yi. “But there are drugs available to control cholesterol that are safe. We hope that when we have completely delineated the mechanisms underlying T cell dysfunction, we can combine cholesterol-lowering drugs with improved immunotherapies so that patients are maximally benefitted.”
Xingzhe Ma, Liuling Xiao, Lintao Liu, Lingqun Ye, Pan Su, Enguang Bi, Qiang Wang, Maojie Yang, Jianfei Qian, Qing Yi. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metabolism (2021) 5:1001-1012.e5. doi: 10.1016/j.cmet.2021.02.015
This research was supported by a Recruitment of Established Investigator award (RR180044) from the Cancer Prevention & Research Institute of Texas in addition to R01 grants (CA239255, CA211073, CA200539 and CA214811) from the National Cancer Institute.
Vandana Suresh, PhD, February 2022