Clinical Research
Semantic dementia study yields new findings
Inflammation research uncovers likely therapeutic target for neurodegenerative aphasia disorder
Inflammation research uncovers likely therapeutic target for neurodegenerative aphasia disorder
3d rendered image of Neuron cell network
Frontotemporal lobar degeneration (FTLD) is the most common form of dementia for people under age 60. The average life expectancy after diagnosis is 13 years and there are currently no treatments available to slow or halt the disease progression.
When this neurodegenerative disorder is characterized by a gradual, progressive aphasia and a decline in the ability to read and write it’s considered a primary progressive aphasia (PPA). People with PPA who progressively lose the meaning of words are considered to have semantic variant PPA (svPPA).
Inflammation is increasingly recognized as a key component of the neurodegenerative process in FTLD, making it a possible therapeutic target for slowing disease progression. This association is clearest among frontotemporal dementia disorders related to TAR DNA-binding protein 43 kDa (TDP-43) pathology.
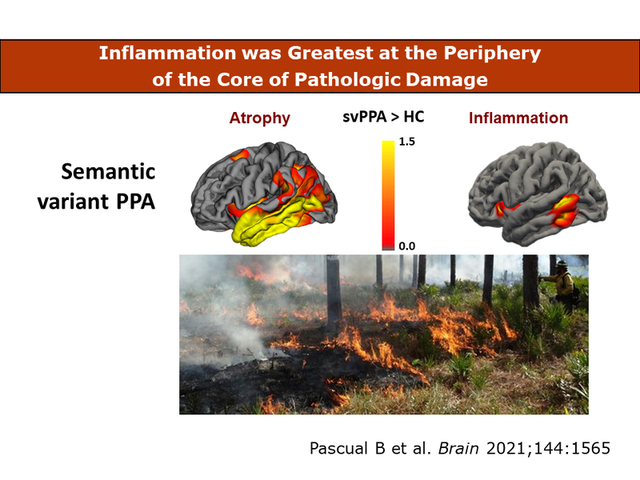
Although researchers have been aware of data linking inflammation in the brain to svPPA, it has been difficult to identify where the inflammation is located. The pathology begins in the tip of the left temporal lobe, where a strong signal appears with the tau PET tracer, 18 F-flortaucipir. The disease, however, is not typically associated with tau but with TDP-43 aggregates.
Amyloid PET, associated with Alzheimer’s disease, usually is negative in svPPA patients. Semantic variant PPA is the only FTLD in which the 18 F-flortaucipir (AV-1451) tau PET is uniformly positive, with a signal to the affected temporal tip. This is an intriguing find because it likely reflects an underlying tissue pathology. It has been put forth that the 18 F-flortaucipir signals in svPPA may reflect TDP-43 type C pathology or binding to monoamine oxidase B (MOAB) receptors.
It also is thought that since the inflammation tracer 11 C-PK11195 has been reported to co-localize with 18 F-flortaucipir in svPPA, the two could be linked. HMRI researchers characterized the topography of inflammation in svPPA more accurately than in previous studies, by using high-resolution PET and the second-generation tracer 11 C-PBR28 with an arterial input function. Two hypotheses were explored: (1) that svPPA was associated with inflammation and (2) that, as in a previous study, the inflammation would co-localize with the 18 F-flortaucipir signal in svPPA and therefore could explain it.
Researchers at Houston Methodist set forth to explore these hypotheses.
A study was designed to study the same svPPA patients with both 18 F-flortaucipir tau PET and 11 C-PBR28. Researchers also wanted to determine whether the pattern of brain inflammation would provide clues to origins of svPPA.
Participants were recruited from the Houston Methodist Nantz National Alzheimer Center and consisted of eight patients meeting diagnostic criteria for mid-stage svPPA18 and 24 healthy matched controls. All patients had a negative amyloid PET scan, which ruled out the diagnosis of Alzheimer’s disease.
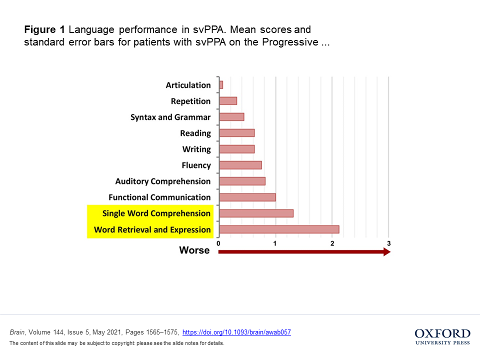
All patients and controls underwent an initial cognitive screening and were also evaluated using the Progressive Aphasia Severity Scale (Figure 1). Patients mostly were impaired in single-word comprehension and naming with spared repetition, articulation, syntax and grammar.
We saw the greatest inflammation in areas of disease progression, rather than in the epicenter of damage, suggesting that inflammation is a key aspect in the progression of svPPA and therefore a likely target for future therapeutic interventions.”

Brain, Volume 144, Issue 5, May 2021, Pages 1565–1575, https://doi.org/10.1093/brain/awab057
Researchers scanned 14 regions, seven by hemisphere, of the brain that were of interest in the patients (Figure 2). The areas were selected a priori from a modified version of Hammers’ probabilistic brain atlas to study the distribution of translocator protein (TSPO) and protein aggregation binding in the patients.
Visual inspection of the 11 C-PBR28 scans of each patient showed elevated signals in the cortex, the highest being along the temporal lobe (left > right), extending posteriorly in the ventrolateral temporal cortex (Fig 3).
Visual inspection of the 18 F-flortaucipir scans showed the elevated signal in the white matter, not in the cortex. The abnormal signal was highest at the tip of the temporal lobe and faded toward the posterior portion of the temporal lobe (Fig 3).
Brain, Volume 144, Issue 5, May 2021, Pages 1565–1575, https://doi.org/10.1093/brain/awab057
The study yielded three original findings: 1.) inflammation, indicated by TSPO expression, was mostly cortical and greatest posteriorly in the temporal lobe, not at the epicenter of cortical atrophy in the temporal tip; 2.) the 18 F-flortaucipir signal was not cortical, but in the white matter, and was greatest at the temporal tip epicenter of the disease; and 3.) the 18 F-flortaucipir signal was not abolished by MAOB blockade with selegiline.
In this study, the inflammation signal was greatest in the cortical ribbon, which could have included the U-fibers of the subcortical white matter. The findings agree with neuropathological studies of FTLD TDP-43 disorders reporting activated microglia to be more prevalent in the cortex and, in TDP-43 type C, immediate subcortical white matter than in the core white matter.
But a finding that has not been reported previously, either in imaging or neuropathological studies, is that in SvPPA neuroinflammation was found to be highest not were the disease began and tissue destruction was highest, but toward the periphery of the affected region, with the pattern of a brush fire (Fig 4). “We saw the greatest inflammation in areas of disease progression, rather than in the epicenter of damage, suggesting that inflammation is a key aspect in the progression of svPPA and therefore a likely target for future therapeutic interventions," said Joseph C. Masdeu, MD, PhD, Graham Family Distinguished Chair for Neurological Sciences.
Although it was clarified that the striking 18F-flortaucipir signal in svPPA is not related to TDP-43, MAO or TSPO binding, researchers were unable to determine the source of this signal, which may represent still unknown neurobiological properties of svPPA.
Belen Pascual, Quentin Funk, Paolo Zanotti-Fregonara, Matthew D Cykowski, Mattia Veronese, Elijah Rockers, Kathleen Bradbury, Meixiang Yu, Mohammad O. Nakawah, Gustavo C. Román, Paul E Schulz, Anithachristy S. Arumanayagam, David Beers, Alireza Faridar, Masahiro Fujita, Stanley H. Appel, Joseph C. Masdeu
Erin Graham, September 2021