Precision medicine
Leading the RNA Revolution
Leading the RNA Revolution
Houston Methodist researchers are redefining the future of RNA vaccines and therapeutics.
Houston Methodist researchers are redefining the future of RNA vaccines and therapeutics.


UPDATES AS OF April 2024
In a recent press release, CEPI announced their partnership with Houston Methodist to further develop the circRNA vaccine platform.
A recent Politico story highlighted Dr. Cooke’s work
Vaccines have been pivotal in protecting human health for decades. And while the SARS-CoV-2 pandemic has been, in turn, terrifying and tragic, it forced the side-by-side evaluation and comparison of multiple vaccine platforms in real time. mRNA vaccines emerged as the clear victor because they are fast, versatile, effective and safe. They encode antigens that are produced in situ, which means high fidelity of their modification, folding and packaging. Because mRNAs are not incorporated into the genome and do not encode an entire pathogen, they have a lower risk profile. In addition, mRNA therapies are faster to develop and simpler to manufacture than traditional vaccine platforms. This success prompted exploration of their potential in other therapeutic areas and the field is growing exponentially.
However, linear mRNAs are degraded quickly in cells, resulting in protein expression lasting only a few days. This limits exposure to the encoded antigen, so mRNA vaccines require at least two initial doses with intermittent boosters for full efficacy. Current mRNA therapies also must use modified nucleosides and incorporate a 5’cap, which greatly increase their cost. In addition, mRNAs can express only a single open reading frame, which constrains antigen design and selection.

John Cooke, MD, PhD
Houston Methodist researchers have overcome these issues and received recent federal and non-government organization funding that will drive their research and advance the field of RNA vaccines and therapeutics. Led by John Cooke, MD, PhD, Chief Translational Sciences Officer, Medical Director, Center for RNA Therapeutics, and Chair, Department of Cardiovascular Sciences, the group has developed proprietary processes in the synthesis, purification, validation and delivery of RNA therapeutics that are poised to change the landscape of RNA vaccines and therapeutics.
VIDEO
Video by Jose Hernandez
A physician-scientist and entrepreneur with 30 years of experience in cardiovascular medicine and stem cell biology, Cooke most recently founded the Center for RNA Therapeutics at Houston Methodist, with a mission to “perform rigorous research that reveals foundational biological mechanisms in RNA biology; translate that knowledge into novel RNA-based products; and to assist other academic groups and small companies develop their great ideas into transformative RNA therapeutics.” The Center received the Vaccine Industry Award for Best Academic Team Research at the 2021 World Vaccine Congress.
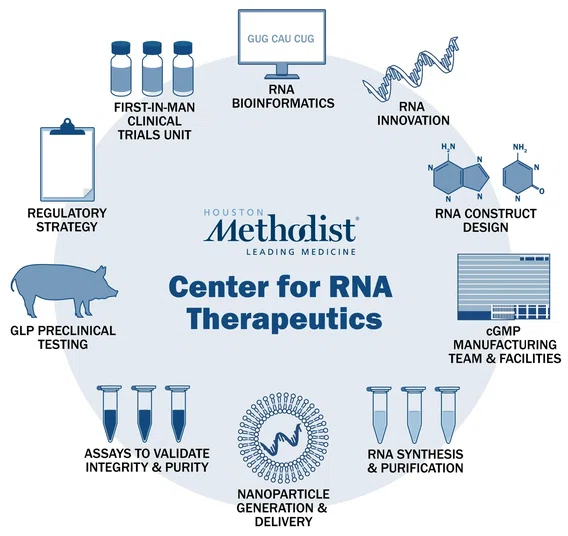
The RNACore plays a crucial role in supporting the center through a renewed multi-year grant from the Cancer Prevention and Research Institute of Texas (CPRIT) and collaborations with industry partners. The core facilitates pre-clinical studies in RNA therapeutics; generates cutting-edge research-grade constructs; and produces RNA therapeutics for clinical trials with licensed, on-site cGMP processes.
Within this framework, Cooke and faculty within the Center for RNA Therapeutics have developed novel methods for generation of more efficient and effective RNA therapeutics that are moving toward clinical applications. Most notable is the technology of Dan Kiss, Assistant Professor of Cardiovascular Sciences and faculty member of the Center. Kiss and his laboratory have developed a novel approach to generate circular RNA that has served as the platform for the Center’s success in securing an international award from The Coalition for Epidemic Preparedness Innovation (CEPI). The Center is also the recipient of new funding from The Biomedical Advanced Research and Development Authority (BARDA). With these awards, the Center is poised to provide leadership in pandemic preparedness and accelerate development of vaccines and medical countermeasures against emerging infectious diseases.
“We're pretty excited about these two funding agencies selecting Houston Methodist for funding. It really does help us accelerate our programs and our progress toward the democratization of RNA therapeutics and improvement of patient care,” said Cooke.
Newly Funded CEPI Grant

CEPI is a global partnership dedicated to expediting the advancement of vaccines against emerging microbes with epidemic and pandemic potential. CEPI’s primary mission is to hasten development of vaccines and other biological countermeasures targeting epidemic and pandemic threats, while ensuring equitable access to those in need. Its vision: a world equipped to generate a new, effective and safe vaccine to a novel pathogen within 100 days.
With funding from CEPI and in collaboration with groups at The University of Texas Medical Branch (UTMB) and Baylor College of Medicine (BCM), Cooke will lead the Center’s effort to refine the novel circular RNA vaccine platform (circRNA) developed in the Kiss Lab; to incorporate antigen designs generated by the Gollihar Lab; to optimize protocols for scaling up production in the RNACore; and to test the efficacy of the vaccines in pre-clinical models of the UTMB group. This platform was built upon the groups’ extensive experience and expertise in manufacturing research quality and clinical grade RNA; antigen selection and protein engineering; the design, generation, and packaging of RNA into lipid nanoparticles; virology and immunology; and vaccine evaluations.
The funded project is expected to demonstrate that their circRNA platform can be used to quickly generate vaccines with characteristics needed to effectively prevent an outbreak from turning into a pandemic, such as multivalency, improved balance of immunogenicity/reactogenicity, thermostability and enhanced response time — all with just a single dose.
Key Houston Methodist researchers on the project include Daniel Kiss, PhD, Assistant Professor of Cardiovascular Sciences, Jimmy Gollihar, PhD, Professor of Pathology and Genomic Medicine, Kristopher Brannan PhD, Assistant Professor in the Department of Cardiovascular Sciences and the Center for RNA Therapeutics and Francesca Taraballi PhD, Assistant Professor of Orthopedic Surgery. Kiss, an RNA molecular biology expert, developed the circular RNA technology. Gollihar, a protein engineer, developed the high-throughput method for finding antigens most likely to result in strong vaccines. Brannan’s STAMP technology to assess RNA binding proteins will guide RNA design and Taraballi, a nanomedicine expert, will lead circRNA encapsulation.
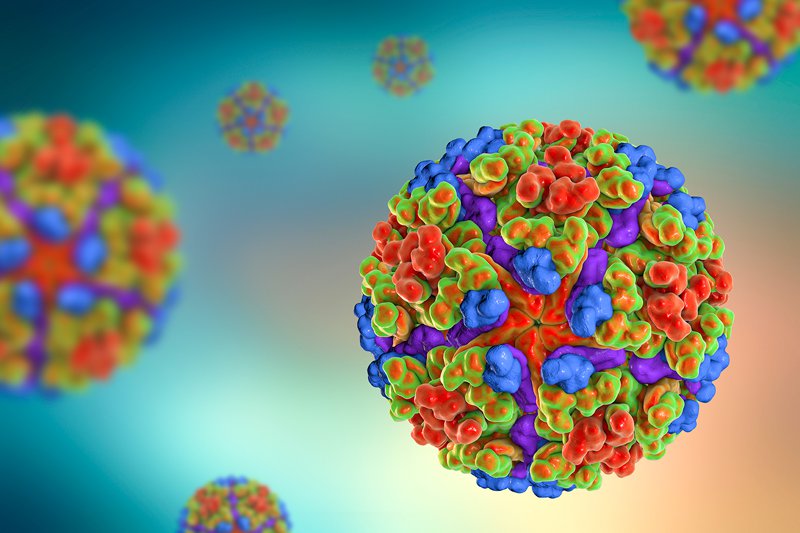
Chikungunya virus, computer illustration. Chikungunya virus is composed of an RNA (ribonucleic acid) genome enclosed in a protein coat, or nucleocapsid (shown here), which is itself surrounded by a lipoprotein envelope. The virus, which is transmitted by mosquitoes, causes a rare form of viral fever. Symptoms include a high fever, rash and arthritic joint pain. Getty Images.
Collaborators at UTMB include Scott Weaver, PhD. Weaver is director, Institute for Human Infections & Immunity, scientific director, Galveston National Laboratory, Chair, Department of Microbiology & Immunology and professor, Microbiology & Immunology and Pathology whose group is internationally recognized for their expertise in virology and vaccine development and testing.
The project is pre-clinical, but the group intends to obtain data needed to design a clinical trial and enable an Investigational New Drug submission to test the cGMP-manufactured circRNA vaccine. Specifically, they will evaluate circRNA vaccine safety; toxicity and reactogenicity; efficacy; and dose range in a pre-clinical model of chikungunya, a mosquito-borne virus causing a spectrum of disease, from self-limited febrile illness to permanent severe disability, congenital anomalies and early death. In the future, a Phase 1 clinical trial would evaluate the safety, reactogenicity and immunogenicity of ascending dosages of the vaccines in young healthy adults with safety as the primary endpoint.
Newly Funded BARDA Contract

BARDA is an Office of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services. BARDA adopts an integrated and systematic approach to develop essential vaccines, drugs, therapies, and diagnostic tools for public health emergencies. This encompasses incidents and attacks related to chemical, biological, radiological and nuclear events, pandemic influenza and emerging infectious diseases.
Recognizing the significance of timeliness in addressing emerging infectious diseases, BARDA invests in novel approaches and technologies to expedite the development of vaccines, diagnostics and therapies needed to combat evolving threats.
Cooke’s team was recently awarded a subcontract from BARDA for a project led by Nature’s Toolbox (NTx) designed to complete development of, and demonstrate, a fully deployable manufacturing unit for production of cGMP RNA vaccines. The result will be a novel RNA manufacturing platform that can be used not only for pandemic response, but a more efficient response to local medical needs.

Founded by biophysical chemist Alex Koglin, PhD, in 2015, NTx is an early-stage life sciences and bioinformatics company based in Rio Rancho, NM. NTx has developed a proprietary, fully recombinant in vitro transcription system (“NTxscribe®”) that is deployable. Product-contact components of the system are single-use and can be supplied pre-assembled and ready to use. With BARDA funding, Cooke's group will complete the development of this cGMP workstation by incorporating encapsulation capability. With preliminary data demonstrating that the system can produce temperature stable, consistent diameter-sized, and efficiently packed lipid nanoparticles; they will optimize protocols for scaling up the process. The formulation system will fit inside the deployable unit and complement the mRNA production.
As a subcontractor on the contract, Cooke’s team brings proven capabilities in cGMP manufacturing and proof-of-concept deployment assessment in a hospital setting.
“The idea is you have a box, you put the reagents in at one end and you get your vaccine out at the other. So, rather than having to ship the vaccine and use cold chain to get it to a low-income country suffering an outbreak, or a theater of war where biological weapons are being used, BARDA could ship the manufacturing unit and the RNA drug could be made on-site,” Cooke said.
He added, “This technology is also going to accelerate the democratization of RNA therapeutics because it will be possible for other hospitals to do what we're doing. The “box” will facilitate hospital-based RNA therapeutics programs for personalized RNA therapy without the need for expensive clean rooms. Our institute went to a lot of expense to generate these clean rooms and it costs a lot to maintain and staff them, but other hospitals won’t need to do that if they have these deployable manufacturing units.”
Combined, these projects further strengthen Houston Methodist’s position on the front lines of emerging infectious disease research and will significantly advance pandemic preparedness and development of countermeasures to a myriad of global public health threats.
Heather Lander, PhD
November 2023
Related Articles