


Outcomes Research
Fast-tracked by the FDA
First plasma therapy given to Houston Methodist COVID-19 patient
Houston Methodist was the first in the U.S. to treat a COVID-19 patient with convalescent plasma therapy .
Houston Methodist received FDA approval on March 28, 2020, to become the first academic medical center in the nation to transfuse donated plasma from a recovered COVID-19 patient into a critically ill patient. The treatment was fast-tracked to the bedside over that weekend, as the death toll in the COVID-19 pandemic had just surpassed 1,000 people across the United States.
In anticipation of the FDA issuing regulatory guidelines, Houston Methodist physician scientists developed a protocol. Principal investigator Eric Salazar, MD, PhD, assistant professor of pathology and genomic medicine, Department of Pathology & Genomic Medicine, in collaboration with James M. Musser, MD, PhD, Fondren Foundation Distinguished Presidential Endowed Chair, and Chair, Department of Pathology & Genomic Medicine, designed and validated a COVID-19 molecular test and were prepared to begin collecting data when COVID-19 patients started arriving.
The Houston Methodist Institutional Review Board reviewed the treatment protocol rapidly, and the Regulatory Affairs and Translational Management team, led by Christina Talley, worked expeditiously to secure FDA approval.
There is so much to be learned about this disease while it’s occurring. If an infusion of convalescent serum can help save the life of a critically ill patient, then applying the full resources of our blood bank, our expert faculty, and our academic medical center is incredibly worthwhile and important to do.
Marc Boom, MD
President & CEO
Houston Methodist

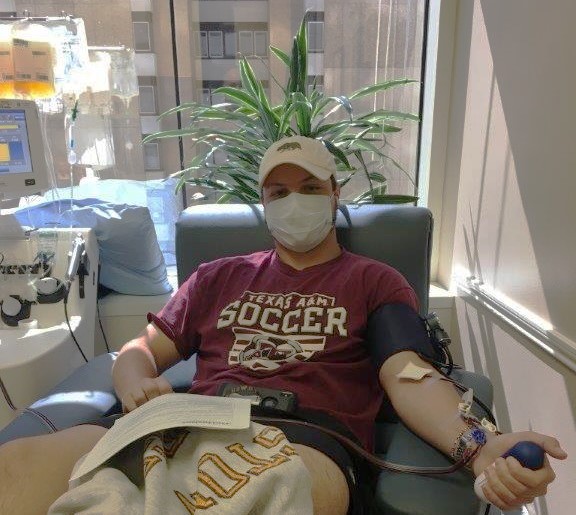
COVID-19 survivor Connor Scott has donated blood plasma multiple times. The antibodies his body made to fight COVID-19 are benefiting patients who are currently fighting the virus.
Known as convalescent serum therapy, the concept dates back more than a century. Similar treatments were used during the Spanish flu pandemic of 1918, a diphtheria outbreak in the 1920s, a flesh-eating bacteria epidemic in the 1930s, and other outbreaks of infectious diseases. While literature abounds on the theory that immunity can be transferred from a healthy individual to a sick individual using convalescent plasma, results have varied. In late March the Journal of the American Medical Association published a description of the treatment for five patients in China, suggesting it was beneficial.
Under FDA guidelines, Houston Methodist’s convalescent serum therapy treatment is classified as an emergency investigational new drug protocol (eIND) that requires FDA approval for each patient infused with donated convalescent serum. Houston Methodist physician scientists will seek additional FDA approval for follow-up studies, possibly a multicenter national trial on the effectiveness of convalescent serum therapy against the COVID-19 virus.
Houston Methodist's initial trial results, published May 13, reported that 19 out of 25 patients improved with the convalescent serum therapy, with 11 discharged from the hospital.
On Friday, March 27, the physician scientists began recruiting blood plasma donors from approximately 250 patients who had tested positive for COVID-19 across the Houston Methodist system. Willing donors each gave a quart of blood plasma in a procedure much like donating whole blood.
On the evening of Saturday, March 28, plasma from the first recovered COVID-19 patient to donate, who had been in good health for more than two weeks, was transfused into a COVID-19 patient at Houston Methodist Hospital.
Plasma from an individual recovered from COVID-19 contains antibodies made by the immune system that may help to kill the virus. Transfusing this antibody-rich plasma into a COVID-19 patient who is still fighting the virus may transform the antibodies into a healing, possibly life-saving therapy.
Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, Eubank T, Bernard DW, Eagar TN, Wesley Long S, Subedi S, Olsen RJ, Leveque C, Schwartz MR, Dey M, Chavez-East C, Rogers J, Shehabeldin A, Joseph D, Williams G, Thomas K, Masud F, Talley C, Dlouhy KG, Valdez Lopez B, Hampton C, Lavinder J, Gollihar JD, Maranhao AC, Ippolito GC, Ojeda M, Saavedra C, Cantu CC, Yerramilli P, Pruitt L and Musser JM. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. The American Journal of Pathology. May 27, 2020. DOI: 10.1016/j.ajpath.2020.05.014.
This study was supported by funding from the National Institutes of Health (grants AI146771-01 and AI139369-01), the Fondren Foundation, the National Institute of Allergy and Infectious Diseases (Contract Number 75N93019C00050), the Army Research Office (Cooperative Agreement W911NF-12-1-0390), Houston Methodist Hospital and Houston Methodist Research Institute.
Patti Muck, March 2020








