Restorative Medicine
Cellular Anti-Aging Therapy
Cellular Lifespan Extension Through Telomere Repair Restores Cell Function and Replication
Hutchinson–Gilford progeria syndrome (HGPS) is a rare disorder of rapid aging. The disease is caused by a mutation that occurs at the time of conception in a lamin A protein called progerin. The syndrome severely disrupts cell architecture and function.
Children with HGPS exhibit growth failure, alopecia, loss of body hair and fat, osteoporosis and aged-looking skin. Patients often succumb to heart disease or stroke and have an average lifespan of 15 years. The rapid aging of these children is associated with accelerated erosion of the telomeres.
Telomeres are structures at the end of a chromosome that gradually shorten as we age. Scientists do not yet know what mechanism causes rapid telomere erosion in HGPS patients, but it is possible is that telomere erosion contributes to rapid aging.

John Cooke, MD, PhD

We are excited to see that many of the features of aging can be reversed at the level of a human cell
using our RNA therapy. The immediate application of our work is to improve cell therapies. Longer term, we believe our RNA therapy will be useful for many age-related diseases.

John Cooke, MD, PhD
Chairman of the Department of Cardiovascular Sciences
Houston Methodist
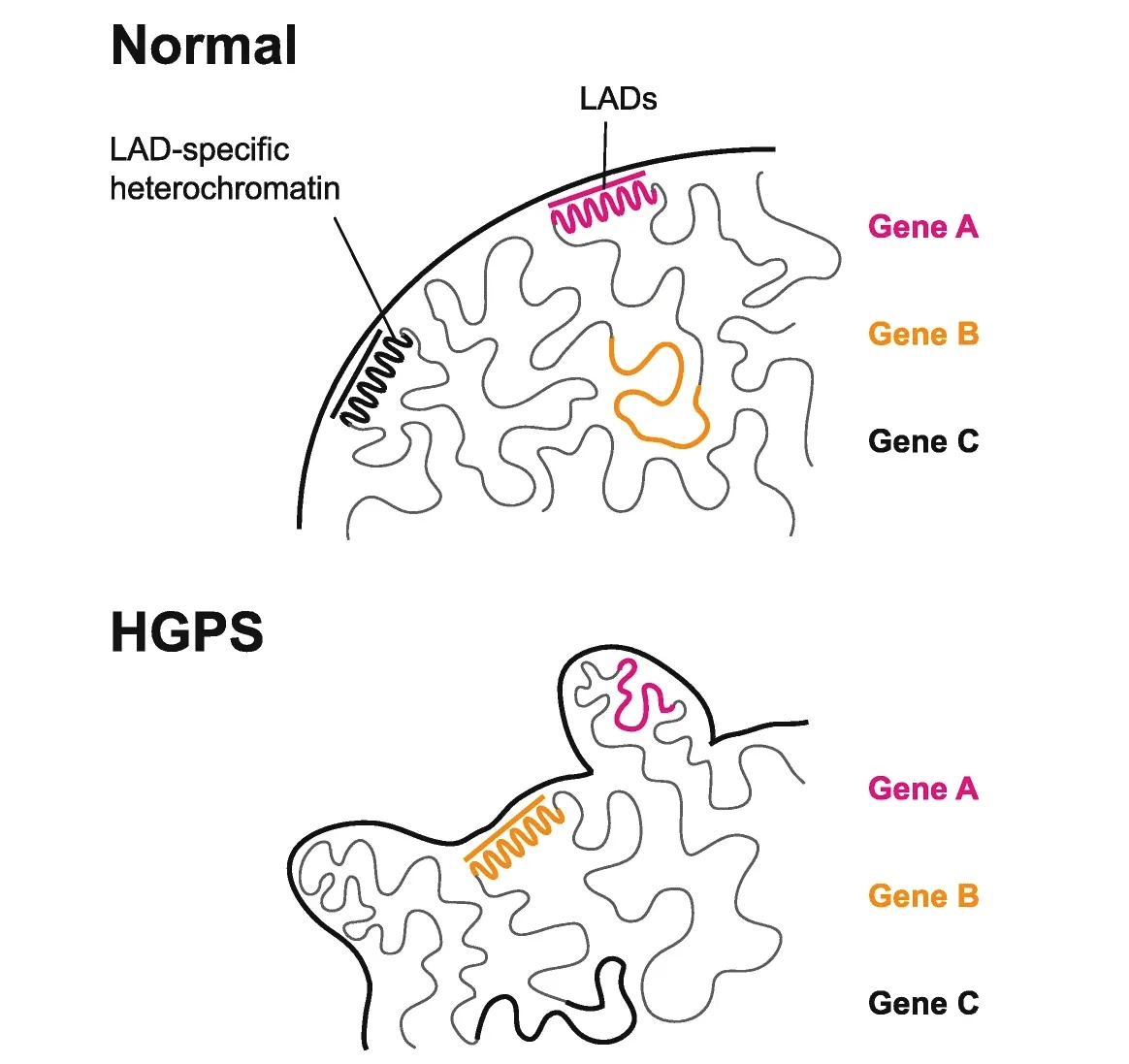
Epigenetic deregulation of lamina-associated domains (LADs)
in Hutchinson–Gilford progeria syndrome (HGPS). Progerin-driven nuclear malformation in HGPS nuclei causes substantial, but
potentially locally stochastic, epigenetic reconfiguration of
LAD-specific chromatin.
A recent study by researchers at Houston Methodist looked at how mRNA encoding human telomerase could treat HGPS.
John Cooke, MD, PhD, and a team of researchers tested the hypothesis that transient expression of purified human telomerase (hTERT) mRNA might restore telomere length, thereby reversing some of the rapid aging in phenotypes of patient cells.
The study revealed that transient expression of hTERT mRNA rapidly restores almost all functions to HGPS cells without transforming cells. HGPS cells treated with hTERT mRNA behaved like young healthy cells, with normal cell growth, function and gene expression.
This study and its proof-of-concept that mRNA technology can reverse aging of cells also led to a collaboration between Houston Methodist and AVITA Therapeutics on an mRNA-based approach to rejuvenate human cells that could be used in multiple areas.
The area of study is a spray-on treatment developed by AVITA Therapeutics that’s used to help older patients with burns that need skin grafting. The spray-on skin is first treated with the Houston Methodist RNA technology to rejuvenate old cells. The rejuvenated skin can then better repair the burn wound.
Mojiri, A., Walther, B. K., Jiang, C., Matrone, G., Holgate, R., Xu, Q., Morales, E., Wang, G., Gu, J., Wang, R., & Cooke, J. P. (2021). Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. European heart journal, 42(42), 4352-4369. https://doi.org/10.1093/eurheartj/ehab547
Erin Graham
December 2022
Related Articles