Multiple myeloma is one of several obesity-related malignancies with multiple studies suggesting an interdependence. However, the precise molecular mechanisms connecting the two remain unclear.
Myeloma is the second most frequent hematologic cancer in the United States, with a median survival of five to seven years, even with state-of-the-art therapeutics. According to the American Cancer Society, there were approximately 32,000 newly diagnosed cases and 13,000 deaths due to myeloma in 2019. The myeloma risk is even greater in obese patients whose body mass index (BMI) exceeds 30 kg/m2. The role of obesity in cancer development is well documented as obesity triggers several mechanistic events in cancer initiation, progression, and response to therapy.
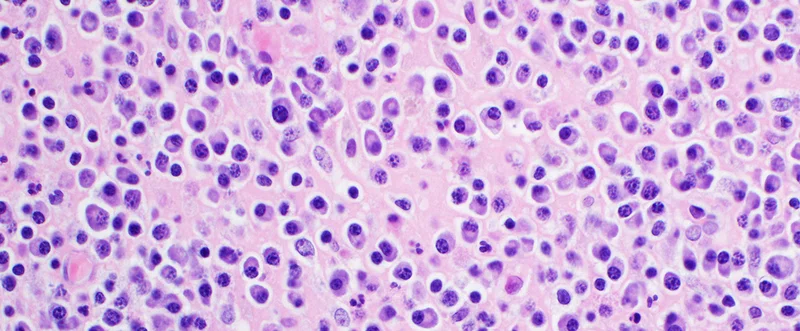
Jing Yang, PhD, associate professor of oncology at Houston Methodist, researched the role of obesity in metabolic variations in myeloma patients by analyzing malignant plasma cells from obese patients. A comprehensive analysis of extensive research experiments using obesity mouse models and myeloma cell lines led Yang to identify the acetyl CoA synthetase 2 (ACSS2) enzyme as a key player linking obesity with myeloma tumorigenesis.
The chief function of ACSS2 is the conversion of acetate to acetyl CoA, a metabolite critical in myriad cellular processes to maintain cellular homeostasis. ACSS2 also comes into play under conditions of metabolic stress in cancer cells where it promotes malignant cell growth and survival. The precise role of ACSS2 in myeloma pathogenesis and obesity-induced myeloma progression was hitherto unknown.
Data from 2011 to 2016 from the National Program of Cancer Registries and Epidemiology and End Results Program indicate a rising trend in the estimated obesity-related myeloma cases. To understand the influence of host obese conditions on myelomatous tumorigenesis, Yang compared a diet-induced obesity mouse model with control mice to estimate the effects of a high-calorie diet on the tumor burden. Both mouse models were intrafemorally injected with murine myeloma Vk12598 cells and results indicated enhanced incidence and development of multiple myeloma under obese conditions. This conclusion is consistent with previous literature suggesting that an adipocyte-rich microenvironment supports tumor growth.
Next, malignant plasma cells from nine newly diagnosed myeloma patients were used to perform microarray gene expression profiling. Comparison of normal weight and obese myeloma patients’ samples led to the identification of 348 genes that are upregulated in myeloma cells of obese patients. Gene ontology analyses further identified inflammatory responses and metabolic processes to be most transformed. Finally, gene set enrichment analyses led Yang to pinpoint the acetyl CoA metabolic process and specifically ACSS1 and ACSS2 to be augmented in samples from obese myeloma patients.
The research study led by Yang produced several significant findings. First, it found an elevated ACSS2 expression in malignant plasma cells from myeloma patients. ACSS2 expression was significantly higher in obese myeloma patients and positively correlated with BMI values. Second, ACSS2 in myeloma cells was found to promote myeloma tumorigenesis via stabilization of oncogenic interferon regulatory factor 4 (IRF4) protein. Third, there was an increased ACSS2 expression in myeloma cells of obese patients in response to angiotensin II, a cytokine secreted by adipocytes. Adipose tissues from obese patients produced more angiotensin II, as compared to non-obese patients. This indicated ACSS2 contribution to obesity-induced myeloma tumorigenesis. Supplemental microarray analyses also suggested higher relative expression of ACSS1 and ACSS2 to be correlated with shorter survival in obese myeloma patients. Lastly, as expected from the mechanistic model, inhibition of angiotensin II-ACSS2 induction prevented obesity-induced tumorigenesis.
Several previously unknown functions of ACSS2 were uncovered in this study. For instance, Yang identified a critical amino acid lysine 399 on IRF4, which is the site of IRF4 acetylation by ACSS2. The lysine 399 residue was found to be evolutionarily conserved across species suggesting this acetylation event to be an important occurrence in vivo. Moreover, acetylation of IRF4 at lysine 399 precludes IRF4 protein degradation by lysosomes - a modulation significant in myeloma development.

ACSS2 expressed by myeloma cells has a function in promoting myelomatous tumorigenesis through enhancing the stability of oncogenic protein interferon regulatory factor 4 (IRF4). Our study thus demonstrates that ACSS2 is a central player in obesity-induced myeloma growth and sheds light on the mechanistic link between obesity and the pathogenesis of myeloma.”
Jing Yang
PhD, associate professor of oncology
Houston Methodist

A dynamic communication exists between adipocytes and myeloma cells. Taken together, the research study led by Yang may lead to several neoteric therapeutic strategies. The first potential therapeutic target is IRF4 since the absence of IRF4 causes myeloma cell apoptosis. The second target is ACSS2 as ACSS2 ablation leads to lower levels of IRF4 proteins, which in turn eliminates myeloma cells. ACSS2 ablation may also be a suitable strategy for other obesity-associated cancers because ACSS2 is highly expressed in multiple tumors. A third potential strategy is intercepting the angiotensin II/AGTR1 signaling to provide therapeutics in myelomas. AGTR1, which is expressed in myriad cancer cells, is the angiotensin II receptor and plays a pivotal role in the cascade to induce ACSS2 expression.
The main limitation of this study is its sole focus on the adipocyte-related activation of ACSS2 and its effect on myeloma pathogenesis. The inherent complexity of obese and tumor microenvironments indicates the presence of additional pathways in the mechanistic control of myeloma tumorigenesis. Obesity-induced inflammatory responses may play a vital role. Although further studies are required in both pre-clinical settings and clinical trials, effective therapeutic strategies using findings in this study are expected in the near future.
Zongwei Li, Huan Liu, Jin He, Zhiqiang Wang, Zheng Yin, Gichun You, Zhiming Wang, Richard E Davis, Pei Lin, P Leif Bergsagel, Elisabet E Manasanch, Stephen T C Wong, Nestor F Esnaola, Jenny C Chang, Robert Z Orlowski, Qing Yi, Jing Yang. Acetyl CoA Synthetase 2: A Critical Linkage in Obesity-Induced Tumorigenesis in Myeloma. Cell Metabolism 33,78-93, January 5, 2021.
This research was supported by the National Institutes of Health/National Cancer Institute (R01s: CA190863 and CA193362) and the American Cancer Society (Research Scholar Grant 127337-RSG-15-069-01-TBG). It was also supported by NIH/NCI P30CA016672 for the Small Animal Imaging Facility, Research Histology Core Laboratory, and Flow Cytometry and Cellular Imaging Facility.
Abanti Chattopadhyay, PhD, July 2021