In 1943, using sausage skins, orange juice cans, a washing machine and other common items to make a device that could clear the blood of toxins, a young Dutch physician named Willem Kolff constructed the first dialyzer.
Kolff began work on an artificial kidney in the late 1930s when he worked in a ward at the University of Groningen Hospital in The Netherlands. There, Kolff watched as a young man died slowly of kidney failure. He decided to find a way to create a machine that would perform the work of the kidneys. About the same time Kolff began his research, World War II erupted.
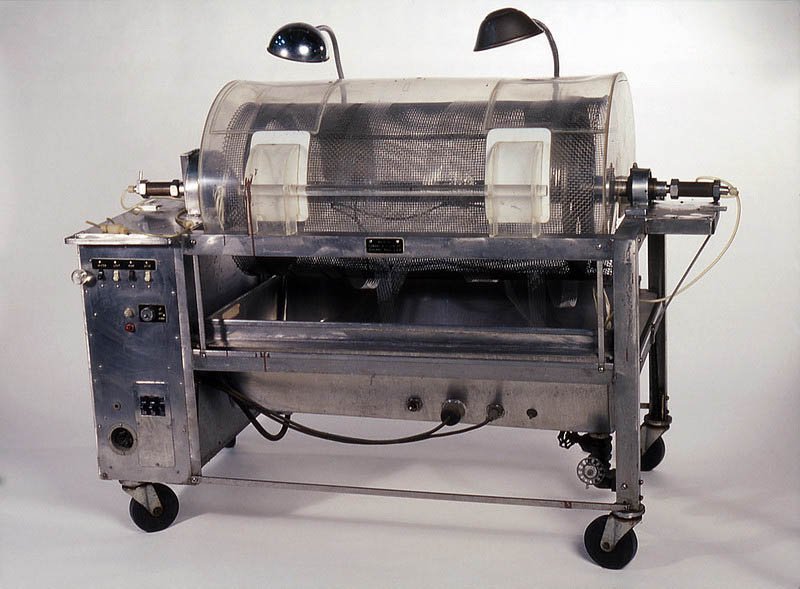
For two years, he treated 16 patients with acute kidney failure with little success until 1945, when a 67-year-old woman in a uremic coma regained consciousness after 11 hours of hemodialysis with Kolff’s dialyzer. She lived seven more years before dying of another ailment.
Kolff’s machine is considered the first modern drum dialyzer and remained the standard for the next decade. After World War II ended, Kolff donated the five artificial kidneys to hospitals worldwide.
In the late 1940s, Kolff came to the United States and continued his research. He gave a set of blueprints for his kidney machine to George Thorn at the Peter Bent Brigham Hospital in Boston. This led to the manufacture of the next generation of Kolff’s dialyzer, a stainless-steel Kolff-Brigham kidney, which paved the way for the first kidney transplant in 1954.
By the end of the 1950s, Kolff’s machine was not seen as a solution for end-stage renal disease failure. Doctors at the time believed patients could not have dialysis indefinitely for two reasons. First, they thought no man-made device could replace the function of kidneys over the long term. Second, a patient undergoing dialysis suffered from damaged veins and arteries so after several treatments, it became difficult to find a vessel to access the patient’s blood.
Belding Scribner, a professor of medicine at the University of Washington, proposed connecting the patient to the dialyzer using plastic tubes, one inserted into an artery and one into a vein. After treatment, the circulatory access would be kept open by connecting the two tubes outside the body using a small U-shaped device, which would shunt the blood from the tube in the artery back to the tube in the vein.
The Scribner Shunt was developed using the newly introduced material, Teflon. With the shunt, it was no longer necessary to make new incisions each time a patient underwent dialysis. Although the Scribner Shunt is no longer used today, it was the first step to improve access methods to the circulatory system.
Today, nearly 500,000 U.S. residents rely on hemodialysis, and almost all undergo at least one vascular access creation surgery to put in an arteriovenous fistula (AVF), a way to reach the blood for hemodialysis. This access allows blood to travel through soft tubes to the dialysis machine, where it is cleaned as it passes through the dialyzer.
Patients receive these access points in one of the following ways:
- Fistula: joining an artery and vein in the arm
- Graft: using a piece of soft tube to join an artery and vein in the arm
- Catheter: soft tube placed in a large vein, usually in the neck

The Center for Rapid Device Translation is proud of the center’s mission to fuel an ecosystem that creates jobs for people,” said Quintana. “It’s not just the growth of a technology that’s important; we are touching the lives of patients, which is at the heart of what we do. We’re pleased that we have facilitated the growth of this technology with VenoStent and are even more excited at the promise of having this technology at Houston Methodist and its acceleration into the clinic is being fulfilled.

Homer Quintana, MCTM
Director, Center for
Rapid Device Translation
VenoStent
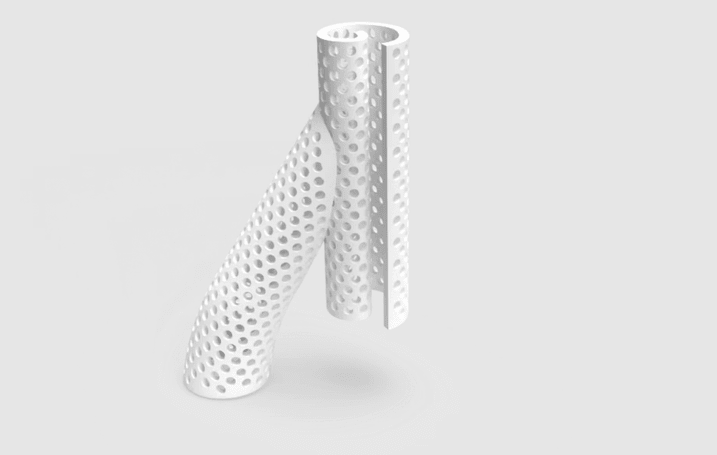
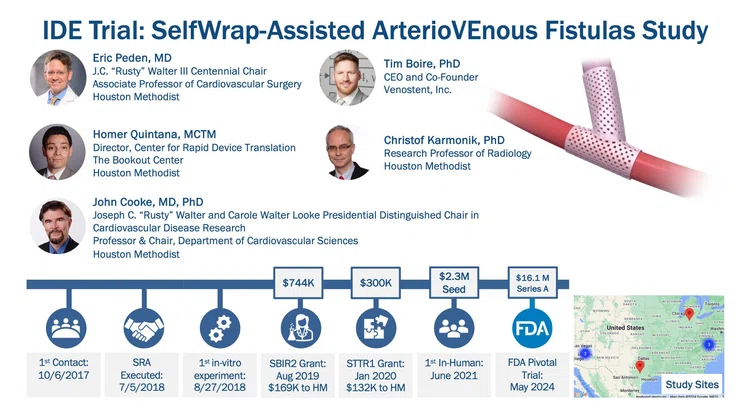
In 2017, VenoStent, a small startup company in Tennessee, approached Homer Quintana, MCTM at Houston Methodist’s Center for Rapid Device Translation (CRDT), with a device it was developing— the SelfWrap — a 3D-printed polymer AVF to improve AVF usability and durability. The CRDT guides clients through safety studies and regulatory approval pathways to bring new medical devices to market. It also provides expertise and study execution to achieve preclinical device validation and opens the door for early phase clinical testing, if necessary.
Quintana contacted Eric Peden, MD, J.C. “Rusty” Walter III Centennial Chair, and Chief of Vascular Surgery, who performs an average of three AVF procedures daily.
“I perform a lot of procedures for people with kidney failure who need dialysis, and maintaining their ability to get effective dialysis is challenging because the outcomes are challenging,” said Peden, Associate Professor of Cardiovascular Surgery. “We're always looking for any innovation in that space to improve their outcomes and, hopefully, their quality of life. We get engaged in many different trials, and that is the reason we engaged VenoStent,”
Peden quickly agreed to help, which was crucial.
“The whole point of the Center for Rapid Device Translation is to be able to facilitate collaborations, and if we don’t have a clinical champion, then it’s a no-go,” Quintana said.
VIDEO
The first step was the submission (in December 2017) and award (in June 2018) of VenoStent’s Small Business Technology Transfer (STTR) Phase I grant from the National Science Foundation (NSF), which afforded an HMRI Core Facility Agreement involving Christof Karmonik, PhD, John P. Cooke, MD, PhD, Peden, and VenoStent to conduct ex vivo fluid dynamic testing. This allowed the team to test the dynamics of the device in a controlled environment with pulsatile liquid flowing through the donor AVFs to simulate blood flow.
Data from these studies was robust enough for the team to successfully submit (in February 2019) and be awarded (in August 2019) a Small Business Innovation Research (SBIR) Phase II grant from NSF. Peden, Karmonik and Cooke were again tapped to help. Peden and his surgical fellows conducted AVF creation surgeries and evaluated flow and vessel caliber via ultrasound. Karmonik assessed the fluid dynamics and AVF anatomies via 4D Flow MRI, and Cooke brought academic expertise with grant submission documentation. VenoStent did the rest.
The total funding received was $744,000. Houston Methodist received $169,000. VenoStent received an additional $500,000 NSF Phase IIB. This funding also facilitated other preclinical studies.
In April 2020, the group received an STTR Phase I grant award of $300,000 from the National Institutes of Health (NIH), with $132,000 to Houston Methodist, to evaluate the impact of the VenoStent technology in a jugular vein to carotid artery bypass grafting model. The results of this study demonstrated the promise of the SelfWrap device to reduce intimal hyperplasia. Additional biocompatibility and degradation studies were also conducted under this grant, further supporting device safety.
SAVE-FistulaS
By June 2021, the team had strong preclinical data supporting the safety and effectiveness of the SelfWrap device, and VenoStent embarked on a first-in-human trial in 20 patients outside the United States, in Asuncion, Paraguay. Three year follow ups have now been completed in this study, and the researchers continue to follow these participants to evaluate outcomes over five years.
SAVE-FistulaS: the SelfWrap-Assisted ArterioVEnous Fistulas Study, a multi-center, single-blind clinical trial involving approximately 200 participants began, in 2024. Peden serves as the national PI, and Houston Methodist will be the lead on all resulting academic publications and the ability to make podium/keynote presentations on patient outcomes at conferences.
Quintana noted that the whole process of taking an innovative technology idea through the safety and efficacy studies and getting it into a Houston Methodist patient in six years is impressive. The 3D-printed polymer technology used in the device will serve as the beachhead for other medical applications.
If the clinical trials are successful and the device receives full FDA approval, VenoStent will mass-produce the product. VenoStent, which started as a startup company with its two founding members, is now a team of more than 20 employees and moved its headquarters to the TMC Innovation Factory, with a recent grand opening coupled with a SAVE-FistulaS Investigator Meeting this past spring.
"The Center for Rapid Device Translation is proud of the center’s mission to fuel an ecosystem that creates jobs for people,” said Quintana. “It’s not just the growth of a technology that’s important; we are touching the lives of patients, which is at the heart of what we do. We’re pleased that we have facilitated the growth of this technology with VenoStent and are even more excited at the promise of having this technology at Houston Methodist and its acceleration into the clinic is being fulfilled.”
Erin Graham
December 2024
Related Articles